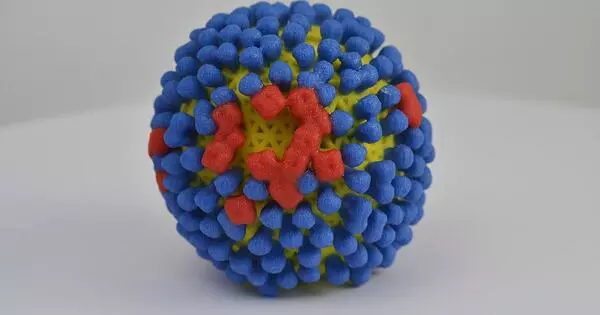
The influenza virus is a highly contagious virus that causes seasonal outbreaks of respiratory illness. Influenza virus replication involves several steps, including attachment, entry, replication, assembly, and release.
Viruses replicate by utilizing the host cell’s molecular repertoire. Researchers from the University of Bonn’s Cluster of Excellence ImmunoSensation2 and Japanese colleagues hope to use this to treat influenza. Prof. Hiroki Kato of the University Hospital Bonn’s Institute of Cardiovascular Immunology discovered a compound that inhibits the body’s own methyltransferase MTr1, limiting the replication of influenza viruses. The compound demonstrated efficacy in lung tissue preparations and mouse studies, as well as synergistic effects with previously approved influenza drugs. The findings have been published in the journal Science.
Viruses require a host cell to replicate. They introduce their genetic information there in the form of nucleic acids such as DNA or RNA. The host cell uses these molecular blueprints to create new viruses. The cell employs a labeling system to distinguish foreign nucleic acids from its own. Own RNA, for example, is identified as non-hazardous by a molecular cap. This allows the immune system to respond to specific threats.
The inhibitor is a synthetic version of the natural product trifluoromethyl tubercidin (TFMT), which is produced by Streptomyces bacteria. We hope that this study will lead to the development of new treatments for influenza.
Prof. Hiroki Kato
The stolen cap
A methylated nucleoside is a small molecule attached to the end of the RNA chain that serves as the molecular cap. When RNA is labeled in this manner, it does not elicit an immune response. If there is RNA in the cell that lacks the cap structure, the immune receptor RIG-I recognizes it and alerts the immune system. To avoid this, influenza viruses have evolved a unique mechanism. They steal the molecular cap from cellular RNA molecules and put it on their own RNA. This is known as cap-snatching.
Influenza requires cellular enzyme for replication
The enzyme MTr1 adds a cap structure to cellular mRNA and thus serves as the cell’s “nucleic acid labeler.” Prof. Hiroki Kato of the University Hospital Bonn’s Institute of Cardiovascular Immunology has now demonstrated how much influenza viruses rely on the function of the enzyme MTr1. “While other viruses, such as SARS-CoV-2, are able to cap their RNA molecules on their own, influenza viruses rely on stealing existing caps,” says Yuta Tsukamoto, lead author of the paper. “If the function of MTr1 is disrupted in the cell, there are no caps available for transfer to viral RNA.” Thus, MTr1 activity is required for the replication of the influenza virus in the cell.
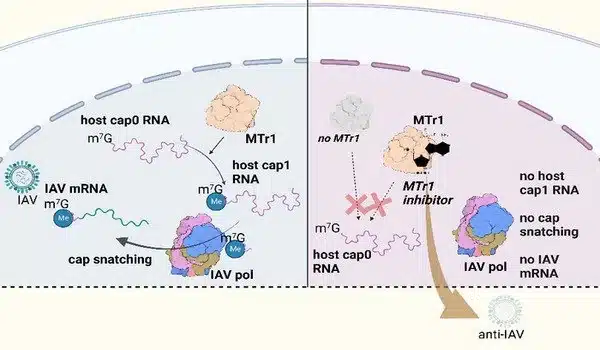
New inhibitor inhibits virus replication
The researchers hope to use this dependence to treat influenza infections. To that end, they sought out inhibitors that specifically target MTr1. The researchers looked into how the substances in infected tissue influence the amount of virus particles produced. This was tested in both mouse models and human lung tissue preparations by the researchers. These so-called lung explants are obtained from patients who have had lung surgery.
“Among thousands of candidates, we were able to identify a molecule that inhibits MTr1 in human lung explants and also in vivo in mice, curtailing influenza replication,” says Prof. Hiroki Kato of the University of Bonn’s Cluster of Excellence ImmunoSensation2.
The inhibitor is a synthetic version of the natural product trifluoromethyl tubercidin (TFMT), which is produced by Streptomyces bacteria. “We hope that this study will lead to the development of new treatments for influenza,” says Prof. Hiroki Kato. The researchers were able to show that TFMT works in conjunction with approved drugs to combat influenza infections in the current study. There was even a clear synergistic effect in terms of the number of virus particles produced in the tissue.