Researchers identified genes to classify diabetic retinopathy factors
Researchers have identified genes that respond differently to high glucose in individuals with and without diabetic retinopathy. In order to explore the genetic mechanisms behind diabetic retinopathy, researchers at the University of Illinois Chicago have also established a novel approach that can be used as a template for the treatment of other diseases. Diabetic retinopathy (DR) is related to both environmental and genetic causes. Several metabolic abnormalities are implicated in its pathogenesis; however, the exact mechanism remains to be determined.
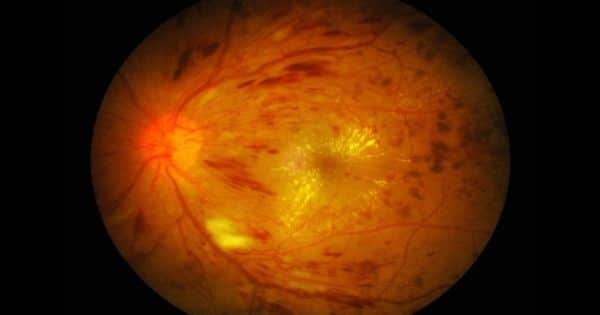
Diabetic retinopathy (DR) is the primary cause of blindness in working-age adults in developing countries. Diabetic retinopathy is characterized by enhanced vascular permeability, haemostatic defects, endothelial dysfunction, increased tissue ischemia, and neoangiogenesis It is well known that diabetes length, impaired glycemic regulation, and hypertension are the main risk factors for developing DR.
In the paper, “Integration of genomics and transcriptomics predicts diabetic retinopathy susceptibility genes,” published in eLife, researchers identified genes that react differently to high glucose in individuals with and without diabetic retinopathy.
Diabetic retinopathy is the leading cause of blindness among individuals between 25 and 74 years of age in the industrialized world. Researchers have identified genes that respond differently in response to high glucose in individuals with and without diabetic retinopathy.
Dr. Michael Grassi, associate professor of ophthalmology at the UIC College of Medicine, his collaborator, Dr. Barbara Stranger of Northwestern University, and their teams set out to find genes that cause diabetic retinopathy, a complication of diabetes caused by light-sensitive tissue damage to the back of the eye – the retina – which results in vision loss.
Grassi has been involved in diabetic retinopathy since he finished his professional training as a retinal specialist. “I encountered two individuals with disparate outcomes, a 19-year-old who had well-controlled diabetes for five years and went blind, and a Vietnam veteran, who had poorly controlled diabetes for over 30 years but had no vision problems,” Grassi said.
Grassi has been looking at the molecular underpinnings of diabetic retinopathy for 10 years. After multiple tries, he eventually settled on a process that culminated in the discovery of genes that raise the likelihood of developing retinopathy.
Grassi and his colleagues have merged many different approaches to classifying a gene known as folliculin or FLCN that raises the chance of developing retinopathy. They started by comparing the levels of gene activation in individuals with and without retinopathy. A group of genes specific to people with retinopathy has been identified. First, they took the genetic markers for this set of genes and discovered that several had been linked with the development of diabetic retinopathy. Finally, they checked whether variations in the levels of any of these genes could induce retinopathy and found that elevated concentrations of FLCN increased the risk of retinopathy.
The study team looked at glucose-induced variations in gene expression in the cell line of individuals with type 1 diabetes, both with and without retinopathy. The method has provided new insights into the disease. The discovery of single nucleotide polymorphisms or PNS correlated with such changes—eQtls (expression quantitative feature loci)—was accompanied by confirmation in separate cohorts. The approach was further improved by FLCN as a mediator of diabetic retinopathy using Mendelian Randomization.
“It has been a challenge to study diabetic retinopathy because it is so heterogeneous. There are so many genetic factors that can contribute,” Grassi said.
In this study, blood-generated cell lines were used in the Diabetes Control and Complications Trial or DCCT, a broad clinical study of diabetic retinopathy. Since the DCCT analysis produced cell lines for each individual, it allowed a thorough characterization of the severity of retinopathy in each individual.
Understanding the molecular reasons underlying diabetic retinopathy will eventually contribute to the advancement of novel treatment and prevention methods for retinopathy. Present quality of treatment includes laser surgery to protect the central portion of the vision, or injections into the eye every four weeks.