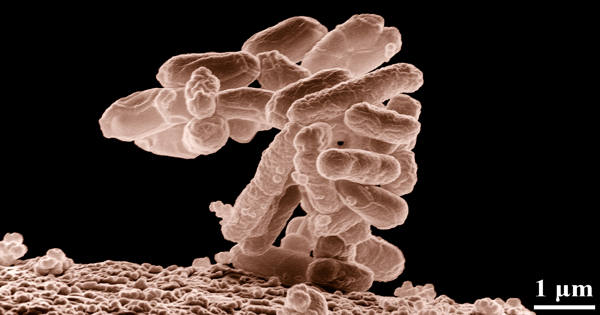
Natural products are produced by microorganisms, such as disease-causing virulence factors or defense substances against predators and competitors. Dr. Robin Teufel and first author Ying Duan from the Institute of Biology II at the Faculty of Biology at the University of Freiburg, in collaboration with researchers from the University of Bonn, have discovered a novel enzyme that is essential for the production of so-called bacterial tropone natural products. The findings were published in the current issue of the Journal of the American Chemical Society.
A natural product is one that is produced by a living organism, according to an organic chemist. There are, however, other organic compounds produced naturally, some of which are of extraordinary complexity and are not primary metabolites. Organic chemists have always been fascinated by the wide variety of these substances, especially those that can be isolated from plants or produced by microorganisms. Many of these compounds, such as alkaloids and mold metabolites, appear to have no discernible metabolic or evolutionary function. In fact, some compounds may be formed as a result of a “metabolic accident” or as by-products of the cellular enzymes’ synthesis machinery.
Researchers discover enzyme prototypes for the formation of ecologically and pharmaceutically important tropone compounds.
When bacteria in terrestrial and marine environments interact symbiotically with plants, algae, or lower animals, they produce tropone natural products, which are thought to be protective substances against microbial pathogens in corals and sponges. The Freiburg researchers then looked into how these bioactive agents are produced by symbiotic bacteria. Teufel and his colleagues discovered an entirely new type of enzyme required for the production of these bacterial tropones.
Natural product biosynthetic pathway enzymes have superior properties and are well suited for use in the synthesis of complex natural product scaffolds (Chemoenzymatic synthesis). Their high selectivity, mild reaction conditions, and ability to be combined to form one-pot multi-enzyme cascades are particularly important. These factors contribute to a reduction in the overall number of steps, for example, by making elaborate protection group strategies and redox transformations redundant. In terms of strategy, their application can lead to more efficient and direct routes to natural products.
The researchers discovered that this enzyme activates oxygen in an unexpected way and incorporates it into a chemical precursor compound. The basic structure of the tropone is generated during the process. The researchers were able to investigate the functions of this enzyme in greater detail using chemical and biochemical methods, elucidating novel intermediates in tropone biosynthesis. “We were able to take an important step toward better understanding the biological production of these important compounds,” Teufel says. “These findings can serve as a basis for better combating certain pathogens in the future or for obtaining novel tropone compounds using biotechnological methods.”
Natural products with medicinal activity have functional group arrays and scaffold architectures that provide advanced platforms for the development of successful new drugs. The investigation of biosynthetic pathways to natural products will aid in the production of target molecules such as commercial drugs and key starting materials for chemical derivatization and semisynthesis. Our lab is interested in the biosynthesis of various pharmaceutically important natural products, such as antimycin-type depsipeptides, the manumycin family, and pyrroloindole alkaloids.
Because of their diverse biological activities, natural products are important sources of pharmaceuticals. Enzymes derived from natural product biosynthetic pathways have emerged as promising biocatalysts for altering the structures and bioactivities of these complex compounds. A large number of enzymes have been harvested in order to create novel scaffolds, large-scale chiral building blocks, and semisynthesis of medicinally relevant natural product derivatives.