Researchers used high-resolution imaging techniques to examine how addictive drugs affect dopamine circuitry in mice. The findings answered old structural questions while raising new ones about plasticity and recovery in the brain.
Researchers use advanced technology and mice to study dopamine neuron structure, addiction, and the brain’s ability to recover. A late 1980s commercial intended to combat drug addiction used a pair of frying eggs as a metaphor for the effects of drugs on the human brain. While researchers have long known that there is a link between drug abuse and negative changes in the brain, it is only now that they can study the alterations in great detail.
Researchers from the University of Chicago and the U.S. Department of Energy’s (DOE) Argonne National Laboratory detailed, for the first time, specific changes that occur in the brains of cocaine-exposed mice using cutting-edge technology.
The study sheds new light on the function of key dopamine neuron structures, which are involved in a variety of functions ranging from voluntary movement to behavior. The findings closed the book on some old questions about how dopamine is transmitted while opening a new one on others. The researchers hope that by continuing their work, they will be able to better understand how certain types of addictions work and, possibly, develop targeted treatments.
The researchers describe how they are expanding on the burgeoning field of connectomics, which is the development of highly detailed and accurate 3D maps of every neuron in the brain and their connections, in a recent paper published in the journal eLife.
The team, for their part, set out to better understand the process by which dopamine is transmitted across neurons, as they do not form traditional physical connections, where signals are transferred across synapses.
We now know that there is an anatomical basis to drug exposure. These animals were given one or two shots of cocaine, and we noticed widespread anatomical changes after two to three days. It’s not as if some molecules are shifting here and there. The circuit is rearranging much earlier and with much less drug exposure than anyone would have thought.
Narayanan Kasthuri
“Evidence suggests that these neurons release dopamine into extracellular space, activating nearby neurons with dopamine sensing receptors,” says Gregg Wildenberg, the project’s lead investigator. “However, because these circuits do not make typical connections, connectomics has had little to say about them, so we wanted to step into this area to see how it actually worked.”
Another motivation for the project was to better understand the role of dopamine in addiction. What anatomical changes in dopamine circuits, if any, are caused by drugs of abuse such as cocaine?
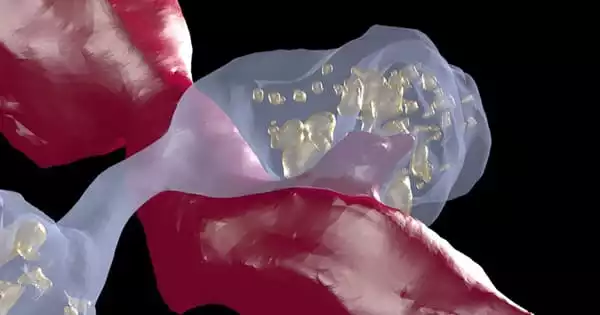
That level of detail necessitated the use of Argonne’s large volume, three-dimensional serial electron microscope. It was a high-powered microscope capable of visualizing the smallest details of the brain, allowing for a more intimate look at the dopamine neurons of cocaine-sensitized mice and control animals.
The team collected approximately 2,000 40 nanometer-thick sections (1mm = 1 million nm) from dopamine-associated sections of the midbrain and forebrain using resources from the University of Chicago.
The SEM generated a collection of 2D, individual images from these samples, totaling over 1.5 terabytes of data. These were digitally reassembled at the Argonne Leadership Computing Facility, a DOE Office of Science user facility, using the visualization cluster Cooley. This process generates a 3D volume that allows researchers to identify and trace different anatomical features of dopamine neurons, which had previously proven difficult.
“The leap of faith in this project was that we would actually be able to detect anatomical changes that might be happening at any point in the brain,” said co-investigator Narayanan “Bobby” Kasthuri. “Could we find anything quantitatively different in this microscopic slice of brain? That is also one of the reasons we chose cocaine: we assumed that whatever was going on was happening systemically throughout the brain.”
The findings established that, with the exception of a few cases, dopamine neurons do not form physical connections. And the latter could imply that dopamine neurons are not all the same; that a different subclass exists that is more prone to making physical connections.
In general, they discovered that small swellings, or varicosities – sites responsible for dopamine release – could be classified into four different types based, in part, on the size and number of neurotransmitter carrying vesicles contained in each varicosity.
Some of these swellings, they discovered, lacked vesicles, prompting some critics to argue that they could not be classified as proper release sites. They believe that the presence of empty varicosities indicates the presence of other molecular components that define dopamine release sites, in addition to the presence of vesicles.
“We propose that these empty varicosities have all the molecular machinery to release dopamine, but it’s also possible that dopamine vesicles are actively shuttled throughout the axon and we just happened to catch a snapshot in time where some are empty,” Wildenberg said.
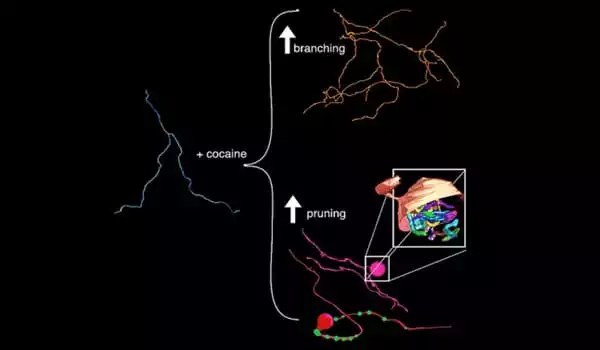
The cocaine portion of the study produced two significant changes, both of which are focused on axons, the ultrathin cables that project from neurons. Axons, like trees, sprout tendrils that branch out to other axons to deliver signals. The team discovered an increase in that branching after exposing the mice to cocaine.
In an unexpected finding, they discovered that roughly half of the axons studied formed massive swellings, or bulbs, at various locations along the axon. The closest analogy to these bulbs can be found in developing animals, at neuromuscular junctions. In some cases, an axon retracts or is pruned, causing it to swell into a large bulb-like structure.
The researchers observed sprouting and retracting in the same axons. According to the researchers, this is the first time this behavior has been observed in the context of a disease model.
“We now know that there is an anatomical basis to drug exposure,” Kasthuri said. “These animals were given one or two shots of cocaine, and we noticed widespread anatomical changes after two to three days. It’s not as if some molecules are shifting here and there” He continued. “The circuit is rearranging much earlier and with much less drug exposure than anyone would have thought.”
While the research has helped to clarify questions about the form, function, and dynamics of the dopamine system, it also raises important new concerns about repeated exposure, addiction, treatment, and recovery.
Based on its plasticity in other areas, can the brain overcome the structural changes caused by addictive drugs? The findings of this study, as well as access to powerful discovery tools, hold the key to answering these types of questions in the future.
This study was published in the journal eLife under the title “Cell type specific labeling and partial connectomes of dopaminergic circuits reveal non-synaptic communication and large-scale axonal remodeling after cocaine exposure.”