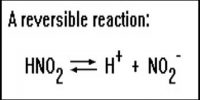Advantages of the Bronsted – Lowry theory:
In 1923, J. N. Bronsted and J. M. Bjerrum in Denmark and T. M. Lowry in England independently proposed a theory known as ‘the proton theory of acids and bases’. According to this theory-
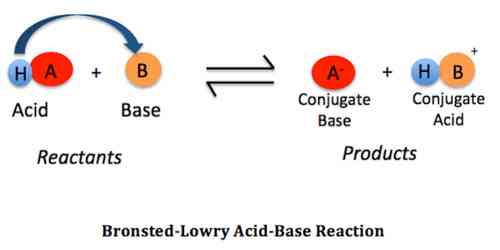
- An acid is a substance – a molecule or an ion – which can donate a proton.
- A base is a substance – a molecule or an ion – which can accept a proton.
It is evident that stronger the acid weaker it’s conjugate base and stronger the base weaker is its conjugate acid. HCl (strong acid) ↔ H+ + Cl– (weak conjugate base)
Advantages of the Bronsted – Lowry theory:
(a) It includes all substances described as acids and bases by Arrhenius. This theory explains the behavior of acids and bases in both aqueous and non-aqueous solvents. It can explain the fundamental character of substances like Na2CO3 i.e. which do not surround OH– group and therefore were not bases according to Arrhenius concept on the basis that they accept protons.
(b) The acid – base reactions are not limited to aqueous solutions only. This concept is not limited to molecules but also covers even the ionic species to act as acids or bases. This is a more universal than Arrhenius theory which includes a large number of acids and bases.
(c) The theory provides a method of comparing the strengths of the ‘strong’ acids and ‘strong’ bases. The usefulness of the Bronsted-Lowry concept in determining the relative strengths of acids and bases can be shown by the following considerations. It can also explain the acid-base reactions in the nonaqueous medium. It explains the acidic nature of CO2, SO2, etc and basic nature of NH3, CaO, etc.
This concept is not limited to molecules but also covers even the ionic species to act as acids or bases. It can also explain the acid-base reactions in the non-aqueous medium.
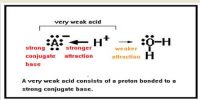
The strength of an acid is a measure of its tendency to give up a proton and the strength of a base is a measure of its tendency to accept a proton. It may be noted that since a strong acid dissociates completely to give protons its conjugate base cannot keep the proton and hence it must be very weak. Similarly, the conjugate acid of a strong base must be very weak.
(i) A strong acid has a weak conjugate base
(ii) A strong base has a weak conjugate acid.
The relative strengths of acids like- HCl, HNO3, HClO4, HBr, H2SO4 cannot be determined in aqueous solutions as they appear to be equally strong in the water. This is because the acids are completely dissociated in water which acts as a strong base. The water here is said to exert a ‘Levelling effect; If, however, these acids are dissolved in a solvent like glacial ethanoic acid, which is not a good proton acceptor, the relative proton donating tendencies become apparent and can be determined by measuring the molar conductance. The strengths of the above-mentioned acids have been found to be in the order:
HClO4 > HBr > H2SO4 > HNO > HCl > HNO3
Limitations of the Bronsted-Lowery concept:
- It cannot explain the reactions between acidic oxides like CO2, SO2, SO3 and basic oxides like CaO, BaO, MgO, etc. which take place even in the absence of the solvent.
- Substances like BF3, AlCl3, etc. behave as acids but they do not have protons to lose or donate.