Although Alcohols (C2H5 — OH) are covalent compounds but they are soluble in water.
Covalent compounds are generally insoluble in water. But some organic compounds like alcohols (R-OH), organic acids (R-COOH), aldehydes (R-CHO), ketones (R-CO-R) are soluble in water. This is due to hydrogen bonding and thus go into solution. For example alcohols remain in aquous solution as follows:
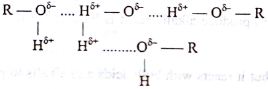
Fig: Formation of hydrogen bond between water and alcohol molecules.