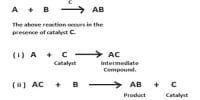
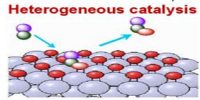
Characteristics of Catalysts
The following characteristics are common to most of the catalytic reactions even though the types may be different.
(a) The catalyst remains unchanged in mass and chemical properties at the end of a reaction. However, the catalyst may undergo some changes in physical appearance and forms. Compact lumps of MnO2 used as a catalyst for the thermal decomposition of KClO3 is found to fall to powder at the end of the reaction. Platinum surface, used as a catalyst’ for hydrogen-oxygen reaction, is found to be coated with a deposit when the reaction is over.
(b) Usually a small amount of the catalyst is sufficient to bring about a large change. This is quite obvious because the catalyst is not present in the product of the reaction, i.e., it is not chemically consumed. Unless a heterogeneous catalyst is poisoned or a homogeneous catalyst is chemically destroyed or lost, the catalysts can be used over and over again bringing about a considerable amount of reaction. In many reactions a trace of the catalyst is sufficient to bring about a large change in a reliantly short time. In some cases, however, a relatively large amount of the catalyst must be present to make significant change in the rate. For example, in the Friedel-Crafts reaction the catalytic activity of solid anhydrous aluminum chloride is increased significantly if it is added in large quantity to the reaction mixture.
C6H6 (l) + C2H5Cl (l) → C6H5C2H5 (l) + HCl (g)
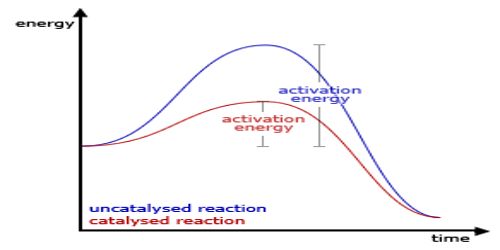
(c) A catalyst does not alter the final position of equilibrium. This is because the catalyst alters the rate of the forward and reverse reactions to the same extent leaving the position of equilibrium unchanged; the time for attainment of the equilibrium is only altered. This condition is in conformity with energy considerations.