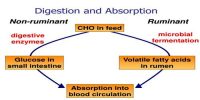
The main component of glass cleaner is isopropyl alcohol. Oil and grease are soluble in it. Detergent molecule has polar sulphate hydrophilic portion at one and a non-polar hydrocabon portion. Hydrocarbon portion are insoluble in water and form an insoluble layer on the water.
When cleaner are spreads on the glass, the oil on grease becomes soluble in non-polar hydrocarbon layer and polar sulphate portion become soluble in water. Detergent molecules surround the oil or grease particle and non-polar part penetrate into these particles and remove from the glass surface.
CH3 — (CH2)10 — CH2 — O — SO3– Na+
Non-polar part poler part
Sodium Lorylsulphate (Detergent)