Some elements (such as carbon and silicon) are most stable when each atom forms several covalent bonds to other atoms. This means that some of the molecules have very large, three-dimensional structures made up of millions of atoms. For example, the pure element carbon can exist as several allotropes (different forms) with different structures and different properties.
One form of carbon is diamond. This has a very strong three-dimensional structure like this, in which every carbon is joined to four others by covalent bonds. This giant molecule can contain billions of atoms. The very regular way that the ions are packed leads to the distinctive regular crystal shape with flat surfaces at fixed angles.
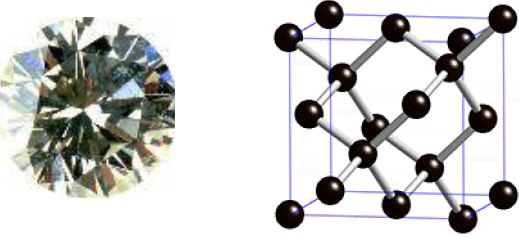
Properties of Diamond
The strong covalent bonds mean that diamond has a very high melting point of 3800°C. The strong bonds and rigid structure makes diamond hard. As the atoms are not arranged in layers, they cannot slide over each other, so diamonds are not malleable or ductile. There are no free electrons, so diamond does not conduct electricity.










