Covalent bonding occurs when too many electrons (typically 3 or more) have to be transferred for ionic bonding to be energetically favourable. Outer octet achieved by electron sharing. Commonly occurs in bonding between the non-metallic elements in Groups 13 – 17. Covalent is found in both elements (e.g. H2, Cl2) and compounds (e.g. CH4, SiC14).
Covalent bonding produces molecules (individual and giant). A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. The shared electrons are attracted to both nuclei of the combining atoms, glueing’ them together.
Formation of a covalent bond between two chlorine atoms
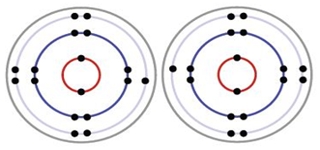
Each Chlorine has 17 electrons.