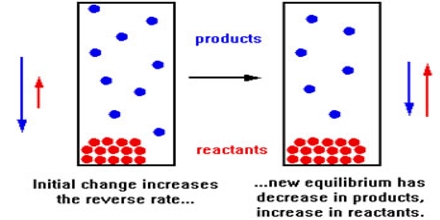
An equilibrium position will always shift if the amount of any substance in the equilibrium mixture is changed. If more reactant is added, or if product is removed, then the equilibrium position will shift to the right i e react to produce more product and less reactant. This is one example of an important general rule known as Le Chatelier’s principle.
Le Chatelier’s principle states that “when a system in a chemical equilibrium is disturbed by a change of temperature, pressure, or concentration, the equilibrium will shift in a way that tends to counteract this changer”.
Consider the following chemical equilibrium:
H2 (g) + I2 (g) ↔ 2HI (g)
Predict the effect on the equilibrium position if
- Some H2 was removed: Equilibrium will be shifted towards the left
- More H2 was added: Equilibrium will be shifted towards the right
- [HI] was reduced: Equilibrium will be shifted towards the right