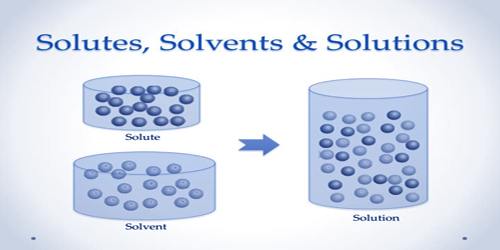
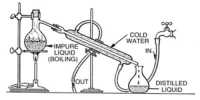
A solvent is a substance that dissolves in a solution which results in a solution. Polar solvents (e.g., water) favor the formation of ions; Nonpollars (e.g., hydrocarbons) do not provide. The solvents are mainly acid, essentially basic, not amphoteric (both) or aprotic (none). The amount of solvent that can dissolve a certain amount of solvent varies with temperature. Common uses for organic solvents include dry cleaning (such as tetrachlorethylene), dilute the paint (such as toluene, turpentine), nail polish government and adhesive solvents (acetone, methyl acetate, and ethyl acetate), spot remover, In detergents (citrus terpenes) and perfumes (ethanol). This homogeneous mixture is completely equal and cannot be physically separated. Heat or any other chemical process must be applied to the solution to separate the solvent and the solvent. Water is the solvent for polar molecules and the most common solvent used by organisms; All the ions and proteins of a cell dissolve in water within a cell. The solids find various applications in the chemical, pharmaceutical, oil and gas industries, including chemical synthesis and purification processes.
Types of Solvents
Molecules generally have two classes of polar and nonpolar. A special case is mercury, whose solutions are known as synthesis; Also, other metallic solutions exist that are liquid at room temperature. Non-polar molecules can fluctuate when charged but do not carry a fixed charge. Both types of molecules can act as solvents as described below.
- Polar Solvents: The polar solvent acts by the action of the positive and negative ends of each atom and the solvent interacting with each other. Since solvents are used by chemists to perform chemical reactions or to monitor chemical and biological events, more precise measures of polarization are required. Most of these systems are sensitive to chemical structure. The positive side of the other solvent molecules is the pull on the negative ions. In this way, the ions are evenly distributed throughout the solvent.
- Nonpolar solvent: Nonpolar solvents work similarly to polar solvents. Non-polar molecules that act as solvents are usually spontaneous dipoles that sometimes form the opposite electric charge between the bonds. The solid polarity water is indicated by its high dielectric constant at 88 (at 0 °C). Solvents with dielectric constant less than 15 are generally considered non-polar. However, polar compounds generally have more powerful interactions between each other with the dipoles of nonpolar molecules. This is because nonpolar and polar solvents such as water and oil are not mixed.