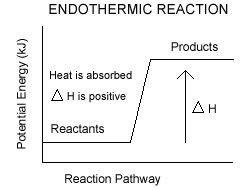
Endothermic reaction: Endothermic reaction is the reaction where heat energy absorbed.
CaCO3 (s) →∆→ CaO (s) + CO2 (g) ∆H298K = + 178 Kj mol-1.
In endothermic reaction the value of ∆H is positive.
Some Common Examples of Endothermic Reaction:
- melting ice cubes
- conversion of frost to water vapor
- evaporation of water
- forming a cation from an atom in the gas phase
- baking bread
- producing sugar by photosynthesis
- separating ion pairs











