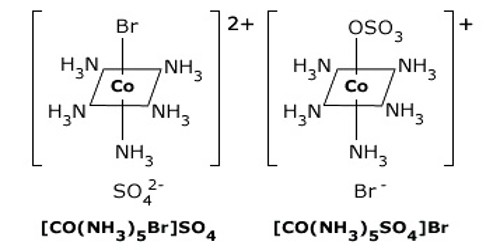
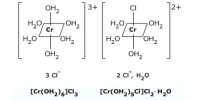
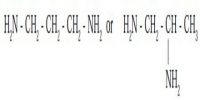Coordination compounds having the same molecular formula but forming different ions in solution are called ionization isomers. This property is known as ionization isomerism.
An example of this type of isomerism is furnished by the red-violet,
Ionization isomers are the compound salts with similar chemical formula but give dissimilar ions when electrolyzed. This phenomenon is known as ionization isomerism. Ionization isomers are indistinguishable except for a ligand has exchanged places with an anion or neutral molecule that was initially outside the coordination complex.
Such complexes consist of a metal, generally, a transition metal, surrounded by species known as ligands. In all complexes, a set of ligands binds to the metal center through the donation of electrons to the metal’s vacant d-orbitals. The ligands which bind in this way are known as inner-sphere ligands because they are bound directly to the metal center.
So, compounds which give dissimilar ions in solution although they have similar composition are called ionization isomerism. The central ion and the other ligands are identical. For example, an octahedral isomer will have five ligands that are indistinguishable, but the sixth will change. The non-matching ligand in one complex will be outside of the synchronization sphere of the other amalgam.
This form of isomerism arises when the counter ion in a complex salt is itself a potential ligand and can transfer a ligand which can then become the counter ion.
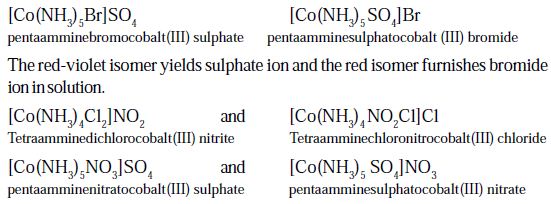
An example is provided by the ionization isomers [Co(NH3)5SO4]Br and [Co(NH3)5Br]SO4.
Another example is, [Pt(NH3)4(OH)2]SO4 and [Pt(NH3)4SO4](OH)2.
Here, isomers happen because of the arrangement of dissimilar ions in a solution. For example,
[PtBr(NH3)3]O2NO2 (anion in solution)
[Pt(NO2)(NH3)]Br (anion in solution)
Ionization isomerism is a form is an isomerism applying to metal complexes. It relates to the way in which the ligands in these complexes bind to the metal center. However, both anions are essential to equilibrium the accuse of the complex. Moreover, the dissimilarity in both isomers is one ion directly attaches to the inner metal but the other does not.