A reaction cascade is a series of consecutive chemical reactions in which the product of one reaction serves as the starting material for the next. This allows for a more efficient process with multiple transformations taking place in a single reaction vessel, reducing the need for isolation and purification steps.
Organocatalysis was used to rapidly generate new fluorinated molecular fragments for drug discovery, according to a team.
Fluorine is uncommon in naturally occurring organic molecules. However, this chemical element is required for the production of pharmaceuticals and agrochemicals. Synthetic chemistry plays an important role in the development of new fluorine-containing molecular fragments. Simple, modular synthesis strategies are extremely valuable.
The strategy has parallels with the art of origami, where you fold complex figures from a simple piece of paper. We can apply this principle to our chemical method.
Louise Ruyet
A team led by Prof. Ryan Gilmour at the Organic Chemistry Institute at the University of Münster has now developed a cascade reaction that allows multiple fluorination reactions to occur sequentially through the generation of reactive intermediates. Using inexpensive organic catalysts and simple starting materials, the researchers demonstrated that the substrate can be manipulated through a type of “molecular origami” to generate a new class of di- and tri-fluorinated molecules in a single operation. The study is published in the journal Nature Communications.
The step-wise construction of complex fluorinated molecules may require multiple purification steps. This has implications in terms of costs, time management, and the generation of waste. Dr Joel Häfliger and Dr Louise Ruyet from Ryan Gilmour’s research group discovered that by fine-tuning the reaction conditions, multiple sequential reactions are possible in a one-pot fashion. In this way, they generated three new classes of complex fluorinated products from simple cyclobutanol derivatives.
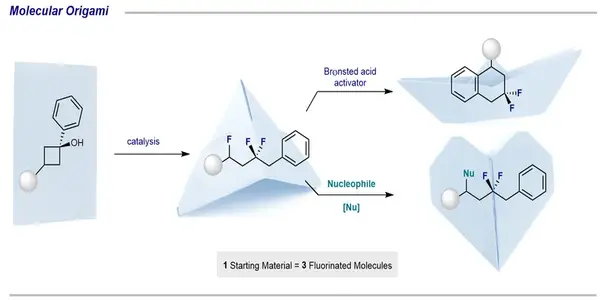
“The strategy has parallels with the art of origami, where you fold complex figures from a simple piece of paper,” Louise Ruyet explains. “We can apply this principle to our chemical method.”
The numerous folding steps represent a series of reactions. “An intermediate compound is created by starting with our piece of paper, the cyclobutanol-derivative. “Depending on the reaction conditions, this compound can be processed into a variety of products,” says lead author Joel Häfliger.
The use of an acidic medium to activate the substrate and produce an intermediate that can be intercepted by a catalytic cycle was critical to the approach’s success. As an example, the researchers created a fluorinated analogue of the agent Nafenopin, which is used to treat hypolipidaemia (a dangerously low lipid level in the blood).
Overall, the development of a reaction cascade for the production of fluorinated molecules is an exciting advancement in the field of chemistry, with potential applications in a variety of industries and scientific research. We can expect to see even more innovative approaches to efficiently and selectively synthesize a wide range of fluorinated compounds as research progresses.