A coronavirus called NL63, which causes the common cold and is also associated with GI symptoms was used in new research using an Intestine Chip to recreate viral infection of the human gut in vitro.
Most of us are familiar with COVID-19’s hallmark symptoms of loss of taste or smell and difficulty breathing, but 60 percent of SARS-CoV-2 patients also report gastrointestinal (GI) symptoms like nausea, diarrhea, and stomach pain. Infection of the gut, which expresses high levels of the ACE2 receptor protein used by SARS-CoV-2 to enter cells, is linked to more severe cases of COVID-19, but the exact interactions between the virus and intestinal tissue in human patients are difficult to study.
While animal models are useful, they do not fully reflect how human organs react to pathogen infection, further limiting our current understanding of how coronaviruses like SARS-CoV-2 affect the gut.
To address this issue, a team of scientists from Harvard University’s Wyss Institute for Biologically Inspired Engineering and several other Wyss partner organizations in Boston used a human Intestine Chip previously developed at the Institute to study coronavirus infection and potential treatments in an environment that more effectively mimics the human intestine than cells grown in a dish.
They infected the Intestine Chip with NL63, a coronavirus that causes the common cold and, like SARS-CoV-2, enters cells via the ACE2 receptor, and then tested the effects of various drugs that have been proposed to treat SARS-CoV-2 infection. They discovered that a drug called nafamostat reduced infection while the drug remdesivir, which has been used to treat COVID-19 patients, did not and actually damaged the intestinal tissue. This new preclinical model, described in Frontiers in Pharmacology, could be used to identify drugs that can target GI symptoms associated with both the common cold and SARS-CoV-2 virus infections in the future.
We were surprised to see remdesivir causing such obvious toxicity to vascular tissue in the Intestine Chip. GI symptoms have previously been reported in remdesivir clinical trials, and this model now provides insight into the underlying causes of those symptoms.
Girija Goyal, Senior Research Scientist
Toxic treatment
The majority of coronavirus in vitro studies are conducted in organoids (blobs of human organ cells grown in a dish), which lack many of the characteristics of living tissues in human organs. Organ Chips address this issue by simulating tissue-tissue contact and other physical conditions that organ cells encounter in the human body. The Intestine Chip is a device the size of a USB memory stick made of a clear, flexible polymer with two parallel channels lined with human blood vessel cells and human intestinal lining cells.
A permeable membrane connects the two channels, allowing cells to exchange molecular messengers and substances to be delivered into the blood via the gut, simulating digestion. The tissues in the Intestine Chip are continuously stretched and released to mimic the rhythmic movements caused by GI tract muscle contractions.
In addition to ACE2, another membrane protein known as TMPRSS2 has been linked to coronavirus infection. The researchers measured the amount of mRNA coding for each protein produced by the cells in the Intestine Chip and discovered that it was significantly higher than in cultured human intestine organoids. They also looked at the RNA molecule repertoires of individual cells and found that the Intestine Chip contained a variety of cell types found in the human gut, such as stem cells, goblet-like cells, and intestinal absorptive cells.
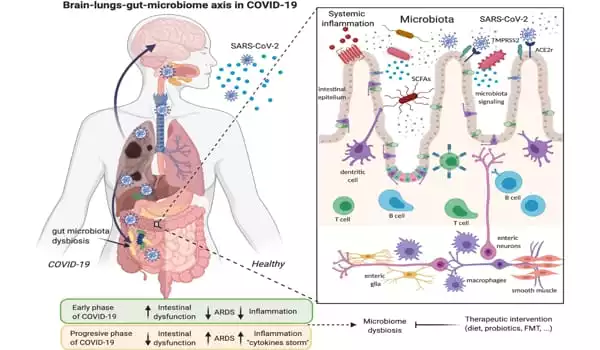
The researchers then injected the coronavirus NL63 into the channel lined with intestinal cells and watched what happened. The Intestine Chip did show signs of infection: the layer of gut cells became “leaky” as the virus compromised the connections between them. To try to cure the infection, the researchers injected nafamostat, a short-acting anticoagulant drug, into the channel lined with blood vessel cells, simulating a human injection.
Nafamostat is a known inhibitor of proteases, a class of proteins that includes TMPRSS2. True to form, nafamostat administration significantly reduced the amount of virus present in the Intestine Chip 24 hours after infection, though it did not restore the integrity of the connections between the cells.
The researchers then repeated the experiment with remdesivir, an antiviral drug that has been granted Emergency Use Authorization by the US Food and Drug Administration for the treatment of COVID-19. Surprisingly, they discovered that remdesivir did not reduce the amount of virus in the Intestine Chip, and it also damaged the cells in the blood vessel channel, causing them to detach almost completely from the channel wall.
“We were surprised to see remdesivir causing such obvious toxicity to vascular tissue in the Intestine Chip. GI symptoms have previously been reported in remdesivir clinical trials, and this model now provides insight into the underlying causes of those symptoms. It may also aid in our understanding of the efficacy and toxicity of other similar drugs “Girija Goyal, Ph.D., a Senior Research Scientist at the Wyss Institute, is one of the study’s co-first authors.
A more complete picture of human gut health
After demonstrating that their Intestine Chip could successfully model interactions between viruses, drugs, and the gut, the researchers tested a number of other oral drugs, including toremifene, nelfinavir, clofazimine, and fenofibrate, all of which have been shown to inhibit infection by SARS-CoV-2 and other viruses in vitro. Only toremifene was found to be as effective as nafamostat in reducing NL63 viral load.
Because the immune system interacts with both pathogens and drugs through the inflammatory response, the researchers introduced a mixture of human immune cells called peripheral blood mononuclear cells (PBMCs) into the Intestine Chip’s blood vessel channel to study this process. They discovered that more PBMCs attached to the blood vessel wall in NL63-infected chips than in uninfected chips, and that the blood vessel cells were damaged. They also discovered that NL63 infection resulted in the release of a slew of inflammatory cytokines, which signal the body to send immune cells to the infection site.
Pre-treating the Intestine Chip with nafamostat before introducing the virus and PBMCs reduced the secretion of some cytokines, but it did not reduce blood vessel damage or completely suppress the inflammatory response. However, pre-treatment with Nafamostat increased the production of an antimicrobial protein known as Lipocalin-2, implying that this type of protein may play a role in the cellular response to coronavirus infections.
“Using our Intestine Chip as a preclinical model, we were able to investigate complex interactions between cells, pathogens, and drugs in the human intestine. We hope it will be helpful in the ongoing effort to better understand the effects of SARS-CoV-2 and identify drugs that could be used to combat future viral pandemics “Don Ingber, M.D., Ph.D., senior author and Wyss Founding Director, is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital, as well as Professor of Bioengineering at Harvard’s John A. Paulson School of Engineering and Applied Sciences.