This new glass has the highest thermal conductivity of any glass material and is the hardest known. The properties of a material are determined by its atomic arrangement. Carbon atoms can combine in a variety of ways, which are classified as either crystalline (forming ordered and repeating lattice structures) or amorphous (appearing more random and disorganized).
The chemical bonds that hold the atoms together determine the hardness of any given carbon material, with two-dimensional bonds producing softer materials such as graphite and three-dimensional bonds producing harder materials such as diamond.
An international research team created a new ultrahard form of carbon glass with numerous potential practical applications in devices and electronics. It has the highest thermal conductivity of any glass material and is the hardest known glass.
Carnegie’s Yingwei Fei and Lin Wang were part of an international research team that created a new ultrahard form of carbon glass with a wide range of potential applications in devices and electronics. It has the highest thermal conductivity of any glass material and is the hardest known glass. Their findings were published in the journal Nature.
Creating an amorphous carbon material with three-dimensional bonds has long been a goal. The trick is to find the right starting material to transform using pressure. Using laboratory techniques to generate extreme pressures to create novel materials or mimic the conditions found deep inside planets.
Yingwei Fei
When it comes to understanding a material’s properties, function follows form. A material’s physical properties are determined by how its atoms are chemically bonded to each other and the resulting structural arrangement, both of which can be seen with the naked eye and which can only be revealed through scientific probing.
Carbon’s ability to form stable structures, both alone and in combination with other elements, is unrivaled. Carbon in some forms is highly organized, with repeating crystalline lattices. Others are more disordered, which is referred to as amorphous. The hardness of a carbon-based material is determined by the type of bond that holds it together. Soft graphite, for example, has two-dimensional bonds, whereas hard diamond has three-dimensional bonds.
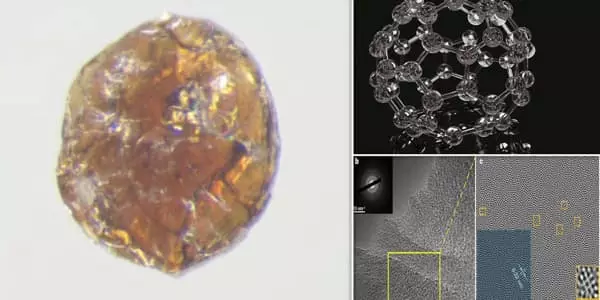
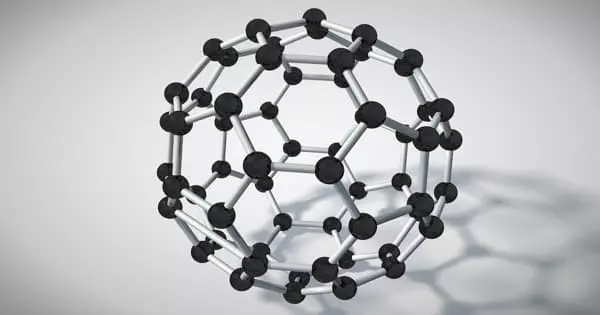
Rather than creating diamond-like glass directly from diamond, the researchers began with fullerene, also known as the “buckyball” due to its resemblance to a soccer ball in structure. Although diamond has an extremely high melting point, fullerene could be heated sufficiently to break bonds in its chemical structure and induce amorphism, then subjected to extreme pressures to transform it into a diamond-like structure. The researchers used a large-volume multi-anvil press to achieve this pressure.
“Creating an amorphous carbon material with three-dimensional bonds has long been a goal,” Fei explained. “The trick is to find the right starting material to transform using pressure. For decades, Carnegie researchers have been at the forefront of the field, using laboratory techniques to generate extreme pressures to create novel materials or mimic the conditions found deep inside planets,” Carnegie Earth and Planets Laboratory Director Richard Carlson added.
Diamond cannot be used as the starting point for the synthesis of diamond-like glass due to its extremely high melting point. The research team, led by Bingbing Liu of Jilin University and Mingguang Yao, a former Carnegie visiting scholar, made their breakthrough by using a form of carbon composed of 60 molecules arranged to form a hollow ball. This Nobel Prize-winning material, known colloquially as a buckyball, was heated just enough to collapse its soccer-ball-like structure and induce disorder before converting the carbon to crystalline diamond under pressure.
The diamond-like glass was created using a large-volume multi-anvil press. The glass is large enough for characterization. Its properties were confirmed using a variety of advanced, high-resolution atomic structure probing techniques.
“The development of a glass with such superior properties will pave the way for new applications,” Fei said. “The use of new glass materials is dependent on the ability to create large pieces, which has proven difficult in the past. Because we were able to synthesize this new ultrahard diamond glass at a lower temperature, mass production is more feasible.”