Crystallization in Solubility
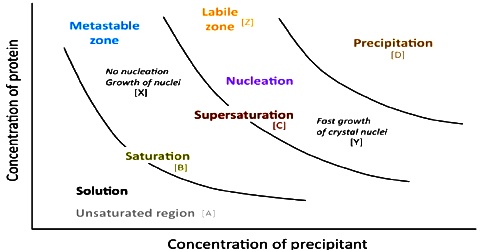
When a solid is added to a liquid and the solution process takes place the concentration of the solute in the solution increases. The particles – ions or molecules – move about at random in the solution and may by chance collide with a crystal of the solute and get attached to it. This process which is opposite to the solution process is called crystallization. As the solute continues to ‘dissolve, more particles enter the solution and the rate at which the particles return to the crystalline state increases. Eventually a dynamic equilibrium is set up between the solute particles in solution and those in the undissolved state, as the rate at which the solid particles dissolve becomes equal to the rate at which the particles return to the solid crystalline state.
Solute (in solution) + water ↔ Solute (solid undissolved state)
When we heat the molecules at high temperature, the intermolecular spaces between them increases as the intermolecular force become due to heat. It helps to adjust more molecules of solute in the increased space. So, solubility of the substances also increases with increase in temperature.