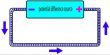
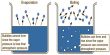
Specific heat capacity of a substance is defined as the quantity of heat required to raise the temperature of 1 kg of the substance through 1K Its unit is J kg-1 K-1.
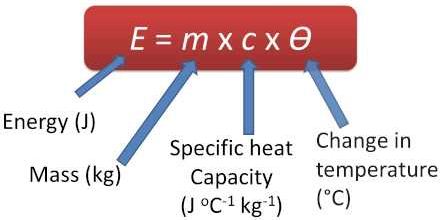
The specific heat of water is 1 cal/gm °C = 4.186 jul/gm °C which is upper than any other universal substance.