Scientists at the University of Adelaide have developed a new simple, low-cost, and quick method for analyzing sulfur isotopes, which can be used to investigate chemical changes in environments such as oceans, freshwater rivers, and lakes.
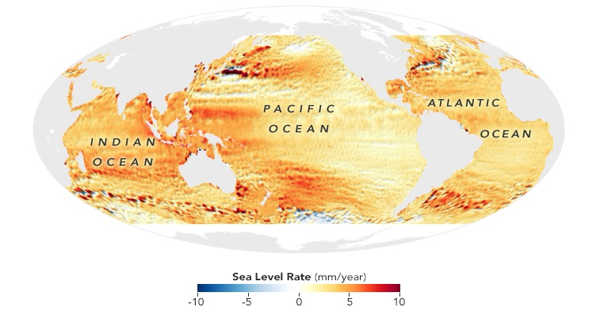
The study, which was published in Talanta, paves the way for new environmental applications of the method, such as tracing the effect of sea-level rise and detecting seawater intrusion into freshwater systems. “Sulfur isotopes can tell us a lot about Earth cycles now and in the past,” said lead author and Ph.D. student Emily Leyden from the University of Adelaide’s School of Biological Sciences.
“Different water sources contain varying levels of sulfur isotopes, and processes within an environment, such as the intrusion of seawater into freshwater systems and the oxidation of acid sulfate soils, can change these ratios. By analyzing sulfur isotope ratios, we can gain important insights into how environments are changing.”
Mass spectroscopy (MS) is the traditional method of measuring sulfur isotopes, in which samples are ionized (split into their ions) and the ions of interest in the samples are measured based on their mass to charge ratio, which varies between isotopes of the same chemical element.
Scientists have developed a new simple, inexpensive and fast method to analyze sulfur isotopes, which can be used to help investigate chemical changes in environments such as oceans, and freshwater rivers, and lakes.
The traditional method has been notoriously difficult because the mass to charge ratio of ions can disperse and overlap, making the results difficult to distinguish. Sulfur is usually only reliably measured if complex chemical purification is performed prior to analysis, which is time-consuming, difficult, and expensive.
As part of Ms. Leyden’s Ph.D. research, a team of researchers from the University of Adelaide’s Metal Isotope Group in the School of Physical Sciences, the School of Biological Sciences, and Adelaide Microscopy collaborated with scientists from Flinders University to develop a novel method for measuring sulfur isotopes using an inductively coupled plasma (ICP) MS instrument.
The new instrument allowed the team to solve the overlapping problem (known as spectral interference) by combining sulfur with another element (in this case, oxygen) to increase the mass to charge ratio, lowering the risk of spectral interference. The sulfur isotopes can then be measured precisely without the need for time-consuming and complex sample purification.
The researchers at the University of Adelaide simulated how the method would work in a real-world scenario by tracing seawater flooding into a variety of coastal environments in South Australia.
The original sulfur isotope of the soil water clearly changed to that of the seawater isotope after flooding. The sulfur isotope ratios of the samples also revealed information about their individual and unique composition prior to seawater flooding. Acid sulfate soil impacts, for example, were detected in two soils, and the signature of historical upstream silver sulfide mining was detected at a site in the upper Onkaparinga River.
According to co-author and Principal Ph.D. Supervisor Associate Professor Luke Mosley of the University of Adelaide’s Environment Institute and School of Biological Sciences, the new method opens up sulfur isotope measurement to a wide range of new environmental applications for scientists from a variety of disciplines.
Scientists at the University of Adelaide have developed a new simple, low-cost, and quick method for analyzing sulfur isotopes, which can be used to investigate chemical changes in environments such as oceans, freshwater rivers, and lakes. The study, which was published in Talanta, paves the way for new environmental applications of the method, such as tracing the effect of sea-level rise and detecting seawater intrusion into freshwater systems.
“Sulfur isotopes can tell us a lot about Earth cycles now and in the past,” said lead author and Ph.D. student Emily Leyden from the University of Adelaide’s School of Biological Sciences.
“Different water sources contain varying levels of sulfur isotopes, and processes within an environment, such as the intrusion of seawater into freshwater systems and the oxidation of acid sulfate soils, can change these ratios. By analyzing sulfur isotope ratios, we can gain important insights into how environments are changing.”
Mass spectroscopy (MS) is the traditional method of measuring sulfur isotopes, in which samples are ionized (split into their ions) and the ions of interest in the samples are measured based on their mass to charge ratio, which varies between isotopes of the same chemical element.
The traditional method has been notoriously difficult because the mass to charge ratio of ions can disperse and overlap, making the results difficult to distinguish. Sulfur is usually only reliably measured if complex chemical purification is performed prior to analysis, which is time-consuming, difficult, and expensive.
As part of Ms. Leyden’s Ph.D. research, a team of researchers from the University of Adelaide’s Metal Isotope Group in the School of Physical Sciences, the School of Biological Sciences, and Adelaide Microscopy collaborated with scientists from Flinders University to develop a novel method for measuring sulfur isotopes using an inductively coupled plasma (ICP) MS instrument.
The new instrument allowed the team to solve the overlapping problem (known as spectral interference) by combining sulfur with another element (in this case, oxygen) to increase the mass to charge ratio, lowering the risk of spectral interference. The sulfur isotopes can then be measured precisely without the need for time-consuming and complex sample purification.
The researchers at the University of Adelaide simulated how the method would work in a real-world scenario by tracing seawater flooding into a variety of coastal environments in South Australia.
The original sulfur isotope of the soil water clearly changed to that of the seawater isotope after flooding. The sulfur isotope ratios of the samples also revealed information about their individual and unique composition prior to seawater flooding. Acid sulfate soil impacts, for example, were detected in two soils, and the signature of historical upstream silver sulfide mining was detected at a site in the upper Onkaparinga River.
According to co-author and Principal Ph.D. Supervisor Associate Professor Luke Mosley of the University of Adelaide’s Environment Institute and School of Biological Sciences, the new method opens up sulfur isotope measurement to a wide range of new environmental applications for scientists from a variety of disciplines.
“With this new method, scientists can easily measure sulfur isotopes in environmental samples after only a simple dilution of the sample of interest,” Associate Professor Mosley explained. “It is especially timely and important given the rapid global environmental change, and the method allows for easier detection of seawater intrusion into freshwater systems as a result of sea-level rise.”