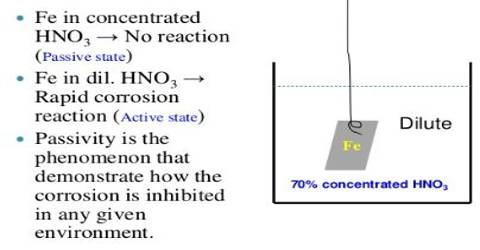
The passivity of Iron:
When a piece of iron is kept for some time in conc. Nitric acid (HNO3), it loses its normal reactivity; it doesn’t react with other substances. This is due to oxidation of Fe into Iron(II, III) oxide (Fe3O4) by nascent oxygen produced from HNO3. The inertness exhibited by metals under conditions when chemical activity is to be expected is called chemical passivity. The following are the common properties of iron. (a) It evolves when hydrogen gas made to react with dilute HCL or dilute H2SO4.
2HNO3 → 2NO2 + H2O + [O]
3Fe + 4[O] → Fe3O4
Apparently, only acids of such concentrations as are capable of yielding nitrogen peroxide in contact with the metal are able to exert a passivifying action. This is further supported by the fact that by passing nitrogen peroxide into those concentrations of nitric acid in which iron is normally active, the metal becomes passive. This passivity of iron is relieved by scratching or heating it in a reducing atmosphere of H2 or it dissolving in diluted HCl. As the Fe2O4 becomes reduced, the iron becomes active again,
Fe3O4 + 4H2 → 3Fe + 4 H2O