Extraction of Iron
Iron is the most widely used metal in the world, mainly in the form of steel. The main
ore of iron is called haematite. It is a mineral composed of iron(III) oxide, Fe2O3.
About 4.12% (or 4.15%) of earth’s crust is Iron. Mainly Fe is extracted from oxide ore. Commercially Fe is extracted from Haematite by three steps: Condensation. Roasting & Smelting.
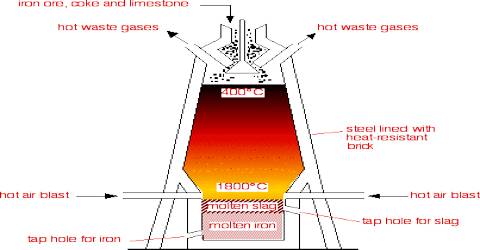
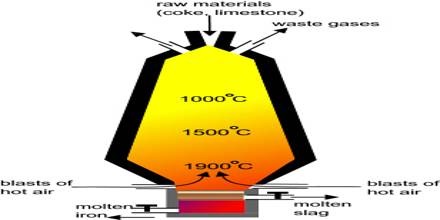
Reduction of Iron (III) oxide (Fe2O3) in the Blast Furnace is the main step of Fe extraction. It is a cylindrical steel-made furnace. It has 3 pasts:
- Upper portion or Stock column
- Middle portion or Boss
- Lower portion or Hearth
The significant reactions occurring within the Blast Furnace can be described via the following steps showing how the reducing agent varies depending on the height in the furnace (i.e. on the Temperature).
At 500 °C
3Fe2O3 +CO → 2Fe3O4 + CO2
Fe2O3 +CO → 2FeO + CO2
At 850 °C
Fe3O4 +CO → 3FeO + CO2
At 1000 °C
FeO +CO → Fe + CO2
At 1300 °C
CO2 + C → 2CO
At 1900 °C
C+ O2 → CO2
FeO +C → Fe + CO
The temperature of the furnace increases from its mouth to the hearth.
- In different parts of blast furnace, the temperature varies from 400°C to 1500°C.
- The temperature of stock column is 400°≈750°C & that of boss is 900°C ≈ 1000°C.
- Near the hearth, the temperature is 1300°C ≈ 1500°C.
- The iron obtained from blast furnace is known as Pig iron/Cast iron, it’s composition is:
C → 2 – 4.5%; Si→ 1-1.5%; Mn → 0.4%.