Efficiency of Carnot’s cycle is independent of the working substance but depends upon the temperatures of heat source and sink. The most efficient heat engine cycle is the Carnot cycle, consisting of two isothermal processes and two adiabatic processes.
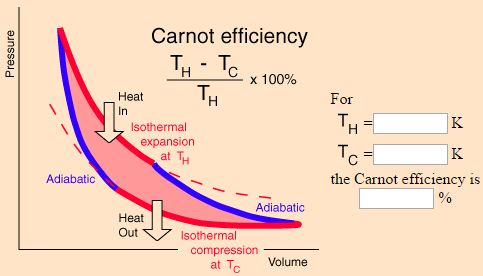
Efficiency of Carnot’s cycle will be 100% if T1 = ∞ or T2 = 0 K. As neither the temperature of heat source can be made infinite, nor can the temperature of the sink be made 0 K, the inference is that the Carnot heat engine working on the reversible cycle cannot have 100% efficiency.











