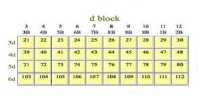
Electrolytic Refining method is used to get metal of high degree of purity. In the electrolytic refining of copper, the impure copper is made from the anode in an electrolyte bath of copper sulfate, CuSO4 and sulfuric acid H2SO4.
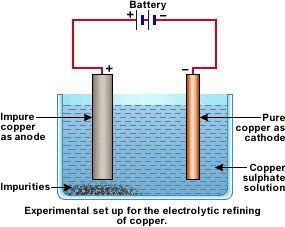
For electrolytic refining of copper,
i) A block of impure copper metal acts as anode
ii) A thin plate of pure copper metal acts as cathode
iii) Copper sulphate solution acidified with sulphuric acid is taken as electrolyte.
When electric current is passed through the electrolytic solution pure copper get deposited on the cathode, impurities settle near the anode in the form of sludge called anode mud.