Should you divide, differentiate, or die? Making decisions at the right time and place defines a cell’s behavior, which is especially important for stem cells in developing organisms. Decisions are based on how information is processed by signaling protein networks.
Christian Schröter of the Max Planck Institute of Molecular Physiology in Dortmund and Luis Morelli of the Instituto de Investigacion en Biomedicina de Buenos Aires (IBioBa) have discovered for the first time that ERK, a key player in stem cell signaling, processes information via fast activity pulses. In stem cell cultures, the duration of the pulsing interval may encode information important for divergent fate decision.
Stem cells go through a series of developmental stages as they develop into the later embryo. The transition between those steps is controlled by signaling molecules that are exchanged between neighboring cells. The fibroblast growth factor 4 is one of the most important signals during early mammalian embryogenesis (FGF4). When it is recognized by a cell, this information is processed by a network of signaling proteins, resulting in a cellular response. The network’s key players, roles, and interactions are now well understood, but little is known about the signaling dynamics. But what does dynamics actually mean, and why is dynamics important?
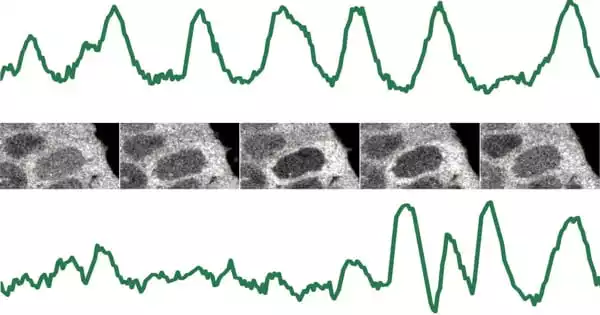
Measuring ERK activity in single stem cells at short timescales is experimentally very demanding and has never been done in such a way before. For the first time, we discovered that ERK activity pulses every six to seven minutes, which is faster than similar signals previously observed in other cell systems.
Christian Schröter
Dynamics determine cell fate
Even though they use the same signal transduction network, two different molecular signals trigger different cellular responses — differentiation and cell growth — in the posterchild example for the importance of dynamics in signal transduction. This is possible because the dynamics that activate the signal transduction system are unique to each of the two molecular signals: Whereas one activates the system for a short period of time, resulting in cell growth, the other activates the same system for an extended period of time, resulting in differentiation.
Thus, signaling dynamics are clearly important in determining the fate of a cell. Many previous studies, however, could only look at relatively slow dynamics that unfolded over hours and were the same in all cells; they were blind to fast dynamics, especially if they differed between stem cells in the same dish.
ERK activity pulses every six to seven minutes
The teams led by Christian Schröter and Luis Morelli were now able to gain a better understanding of the fast signaling dynamics in stem cells. The scientists were able to measure the activity of the major signaling protein ERK in real-time by incorporating a fluorescent sensor into living stem cells. “Measuring ERK activity in single stem cells at short timescales is experimentally very demanding and has never been done in such a way before.”
For the first time, we discovered that ERK activity pulses every six to seven minutes, which is faster than similar signals previously observed in other cell systems. “In single cells, pulses occurred frequently and very regularly one after the other, but pulsing patterns were strikingly different between individual cells,” says Christian Schröter. The researchers also noticed that as the FGF4 signal increased, the number of pulses increased when summed across many cells, despite the fact that the durations of single pulses did not change with FGF4.
Interdisciplinary approach — Intercontinental collaboration
“This type of data, and its role in cell signaling, is extremely difficult to interpret. And it was at this point that our expertise came into play “According to Luis Morelli, a long-standing collaboration partner and group leader at the IbioBa, a Max Planck Society partner Institute. “To describe the dynamics in time series, we needed to develop a new theoretical approach. We discovered that the duration of the pulsing interval could encode information because we could find pulses and silence. This new dynamic feature is known as intermittent oscillations.”
“Oscillations are becoming more and more recognized as a feature of signaling processes. We hypothesize that the intermittent oscillations we discovered in stem cells function as a type of morse code that encodes differentiation information. Presumably, the transition from pulsing to silence is critical. The question now is, what do the dynamics tell us about the organization of signaling in stem cells? How do cells read oscillations, and how do they affect cell behavior? I am convinced that close collaboration between experimentalists and theorists will be required to one day unravel the origins and functions of this new dimension in stem cell biology “Christian Schröter expresses himself.