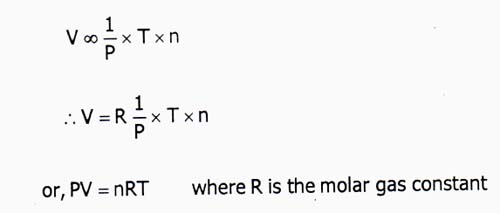Let pressure, volume and temperature of a fixed mass of gas be P, V and T respectively.
Boyle’s Law: At constant temperature, the volume of a fixed mass of gas is inversely proportional to its applied pressure.
Mathematical form:
V ∞ 1/P when T is constant ………………(i)
Charle’s Law: At constant pressure, the volume of a given definite mass of a gas increases or decreases by 1/273 part of his volume at 0°C for each degree Celsius rise or fall in temperature.
Alternative form: The volume of a given mass of gas directly proportional to its absolute temperature when pressure remains constant.
Mathematical form:
V ∞ T when P is constant ……………….(ii)
Avogadro’s Hypothesis: At same temperature and pressure, the equal volume of all gases [elemental or compound] contains the equal number of molecules. It can also be stated as a volume of gases at constant temperature and pressure is directly proportional to their number of moles.
Mathematical form:
V ∞ N when P and T are fixed,
⇒V ∞ n ………………..(iii)
Again, n ∞ N where n=number of moles and N=number of molecules
From equation (i), (ii) and (iii) we get,
V ∞ (1/P) × T × n
⇒ V = R(1/P) × T × n
or, PV = nRT, Where R is the molar gas constant.
This is the ideal gas equation.