Gas is a physical state of matter at normal temperature and pressure. In this state the translational motion of molecules is greater than their intermolecular attraction. For example at normal temperature and pressure hydrogen is a gas, H2.
The characteristics of Gases are given below:
- Structure of Gases: Gas has no structure. It is composes of very tiny elastic spherical particles called molecules, which move high kinetic velocity randomly in straight lines.
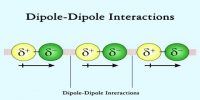
- Intermolecular force and shape: The intermolecular space between the molecules is very high and has less intermolecular attraction.
- Mixibility: When different gases which do not react chemically are brought in contact in any proportion they intermix almost immediately or diffuse rapidly into each other forming homogeneous mixture.
- Uncertainty of Shape and Volume: Gas has neither definite shape nor volume and tends to fill up whole volume of the container in which it is kept.
- Pressure of Gases: Molecules of gas move in straight lines at a very high velocity until they collide with each other or with the wall of the container. The force applied due to this collision on unit area of the wall of the container is known as the pressure of the gas, i.e. P = F/A
- Compressibility and expansibility: Since the gas molecules are far apart from each other, they can be compressed to a greater extent. Again as the intermolecular attraction between the molecules are less so they can easily expand.
- Effect of temperature and pressure on gases: When the pressure on a gas is increased, the intermolecular space between its molecules get reduced as a result volume decreases. again, when the temperature is increased and pressure is kept constant then the volume increases.
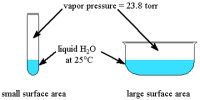
- Relative volume: The relative volume of gas with respect to other states is very high. As a result, density is low.