Task: To produce pure water from saline water.
Required accessories: 1 distillation apparatus, 500 milliliters of saline water.
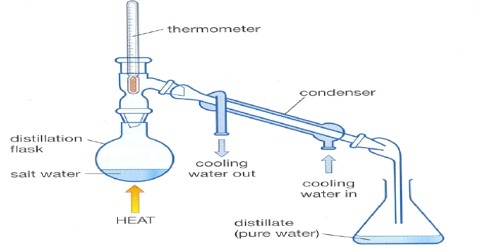
Procedure: Place the distillation apparatus (as in figure) and take saline water in the receiving flask (round bottomed) (1). Connect the inlet tube (4) of the apparatus with a water tap and open it to flow water. Connect a plastic pipe with the outlet tube (5) and keep it in the basin. Now continue to apply heat by electric heater. If there is no electric heater system, heat can be applied by a spirit lamp or any other way. Observe the temperature in the thermometer of the apparatus. When the collection of vaporized water through the narrow tube of the condenser (2) in the spherical flask (6) begins, start waiting. When the amount of saline water in the spherical flask is (round bottomed) (1) reduced by three-fourth portion then stop heating.
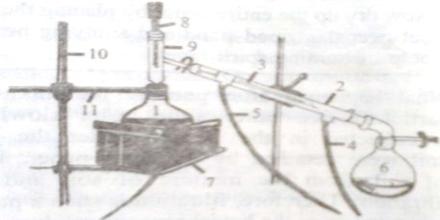
Destillation Apparatus
Analysis
What happens after applying heat? The temperature of the thermometer increases slowly and at 1000 Celsius. The water begins to boil and converts to steam. That steam, when enters into the condenser, then convene into liquid. This process of converting steam (vapor) into liquid as called condensation. The condensed water continues to accumulate in the acceptor flask in drops. The collected water is the pure water.
Hence we see that to make pure water from any mixture of water, we are to apply two Processes. One of this is vaporization and the one is condensation.