Complex Reactions
Complexities may arise as other reactions involving the products of a simple reaction which makes the study of the kinetics of such reactions complicated. Brief discussions of Reversible reactions are given here.
Reversible reactions
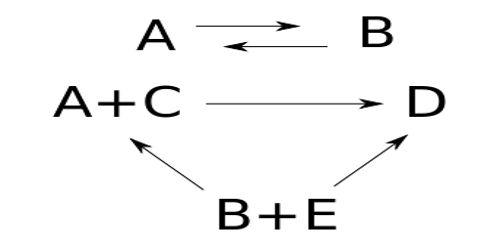
A number of reactions, both in solution and in the gas phase, are reversible, i.e., the forward reaction is opposed by the reverse reaction. Such reactions are the special type of simultaneous reactions. Consequently, in the treatment of kinetic data, the factors due to the reverse reaction must be considered. In reversible reactions, a number of possibilities arise and for the sake of brevity a summary table is given below for the various possibilities –
Table: Rate of Expression for opposing Reactions
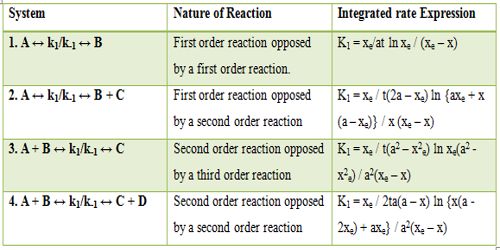
In Table, xe corresponds to the concentration of a suitable product at equilibrium and all a terms correspond to the initial concentration and x is the concentration of the product at any time t.
Examples of reactions from 1 to 4 in the table are:
(i) mutarotation of π- bromonitrocamphor in chloroform solution.
(ii) decomposition of certain alkyl ammonium halide to a tertiary amine and an alkyl halide in solution, thermal decomposition of ethyl bromide in the gas phase.
(iii) isomerisation of alkyl ammonium cyanide to the corresponding substituted urea in aqueous solution.
(iv) decomposition or the formation of hydrogen iodide respectively.