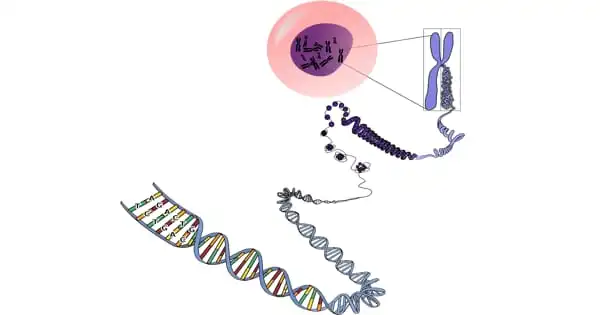
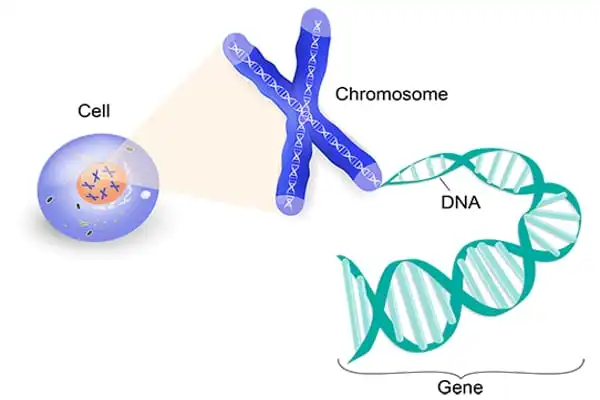
Each human cell has the instructions required for the cell to perform its function. These instructions are comprised of DNA (deoxyribonucleic acid), which is densely packed into structures known as chromosomes. Each chromosome contains thousands of segments known as genes. Genes are inherited from one’s biological parents. They contain data that determines characteristics such as eye color and height. Genes also play a function in the health of the body’s cells.
Diseases can be caused by genetic mutations (permanent changes in one or more particular genes). If a person inherits a genetic mutation that causes a disease, he or she will likely obtain the disease’s DNA strand. Inherited genetic illnesses include sickle cell anemia, cystic fibrosis, and some cases of early-onset Alzheimer’s disease.
While the word “mutation” may conjure up frightening images, a mutation in brain immune cells plays a good effect in preventing patients against Alzheimer’s disease. Biologists at the University of California, Irvine have now found the mechanics underlying this critical activity. Their research was published in the journal Alzheimer’s and Dementia.
The study focused on a variation of the PLCG2 gene, which encodes instructions for the production of an enzyme vital to brain immune cells known as microglia. “Recently, the mutation known as P522R was proven to lessen the chance of acquiring late-onset Alzheimer’s,” said Hayk Davtyan, Ph.D., senior researcher in the laboratory of Mathew Blurton-Jones, professor of neurobiology and behavior, where the study was done. The project was led by assistant project scientist Christel Claes, Ph.D., the paper’s first author.
For the first time, our findings demonstrated that the P522R variation boosted expression levels of multiple microglial genes that are lowered in Alzheimer’s patients. This is some of the first data to explain how this protective mutation may lessen the risk of Alzheimer’s disease.
Hayk Davtyan
The researchers used CRISPR gene editing technology to create the protective mutation in human stem cells before implanting microglia produced from those stem cells into humanized rodent models of Alzheimer’s disease.
Alzheimer’s disease is a brain degenerative illness that causes dementia, which is a progressive loss of memory, judgment, and capacity to operate. This disorder commonly manifests itself in adults over the age of 65, however less prevalent types of the disease manifest themselves earlier in adulthood.
The most prevalent symptom of Alzheimer’s disease is memory loss. Forgetfulness may be minor at first, but memory loss worsens over time to the point where it interferes with most parts of everyday life. Even in familiar surroundings, a person suffering from Alzheimer’s disease may become disoriented or confused. Routine duties such as meal preparation, laundry, and other domestic chores might be difficult. Furthermore, it may become difficult to distinguish and name individuals and items. Affected persons are more reliant on others for assistance with clothing, eating, and personal care.
“For the first time, our findings demonstrated that the P522R variation boosted expression levels of multiple microglial genes that are lowered in Alzheimer’s patients. This is some of the first data to explain how this protective mutation may lessen the risk of Alzheimer’s disease” Davtyan stated this. The variation also boosted the number of T-cells, or white blood immune system cells, in the brain, implying that it may activate other crucial components of immune function.
The findings will aid in the design of future studies to better understand how microglia and T-cells interact to slow the progression of Alzheimer’s disease. “Therefore, the next step could be to identify drugs that can safely increase the activity of the PLCG2 enzyme and further promote protective microglial functions,” he said.
Christel Claes, the study’s first author, wonders if a TREM2 boosting antibody, such as the one now being tested in a Phase 2 clinical trial by Alector (AL002), could provide similar protection in Alzheimer’s patients as the P522R variation.
“It is well known that the PLCG2 P522R mutation increases TREM2 downstream signaling, which is an AD risk variant, so studying the effect of TREM2 stimulating antibodies on microglia-T cell crosstalk will be very interesting. This is what drives us as neuroscientists: studies like ours pave the way for new strategies to treat or prevent this disease that is wreaking havoc on humanity” Davtyan stated.
Mutations in these genes cause the generation of aberrant proteins linked to the disease. Each of these mutations contributes to the breakdown of APP, a protein whose specific function is unknown. This breakdown is part of a process that results in the formation of damaging forms of amyloid plaques, which are a hallmark of Alzheimer’s disease.
A BrightFocus Postdoctoral Fellowship, the Cure Alzheimer’s Fund, the National Institutes of Health, and the National Institute on Aging all contributed to the project’s success.