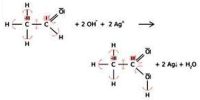
A salt is formed by the neutralization of an acid by a base. There are different types of salts. They are:-
- Simple salt
- Molecular (or) addition compounds
Simple salt
A simple salt is formed by the neutralisation of an acid by a base.
KOH + HCl → KCl + H2O
Normally, a simple salt ionises in water and produces ions in solution. The solution of the simple salt exhibits the properties of its component ions.
Molecular (or) addition compounds
i) Double salts
These are molecular compounds which are formed by the evaporation of solution containing two (or) more salts in stoichiometric proportions. Hence the molecular compounds which dissociate in solution into its constituent ions are known as double salt. Double salts retain their properties only in solid state.
They are also called as lattice compounds.
Example:
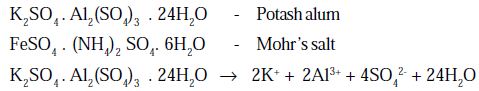
The double salts give the test of all their constituent ions in solution.
ii) Coordination (or complex) compounds
Coordination compound is ‘a compound formed from a Lewis acid and a Lewis base’. The molecular compounds, do not dissociate into its constituent ions in solution are called coordination compounds.
Example:
(Ferrous cyanide) Fe(CN)2 + 4KCN → Fe(CN)2 . 4KCN (or) K4[Fe(CN)6]
Fe(CN)2 . 4KCN ↔ 4K+ + [Fe(CN)6]4- (Complex anion)
In K4[Fe (CN)6] the individual components lose their identity. The metal of the complex ion is not free in solution unlike metal in double salt in solution.