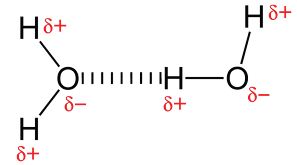
H has the lowest electronegativity of any of the non-metals, so all bonds involving H are very polar. Thus compounds with O-H, N-H or F-H will show significant dipole-dipole attraction. These are known as hydrogen bonds. Remember though that these intermolecular forces are still much weaker than full covalent bonds.
Although non-polar molecules have no permanent dipole, it is possible to have a small temporary dipole for an instant when the electron cloud happens to be more to one side of the molecule than the other. This temporary dipole can attract or repel the electron cloud of a neighbouring atom, and induce another temporary dipole there. These temporary dipoles will spread continually throughout any substance. These cause weak attractive intermolecular forces known as London dispersion forces. The size of these forces increases for larger molecules with more electrons, or as the molecules get closer together- i.e. at low temperatures or high pressures.
The evidence for London forces is that even elements that are monatomic or consist of diatomic molecules (in which there can be no permanent dipole) do liquify at cold enough temperatures- so there must be some attractive forces between them.