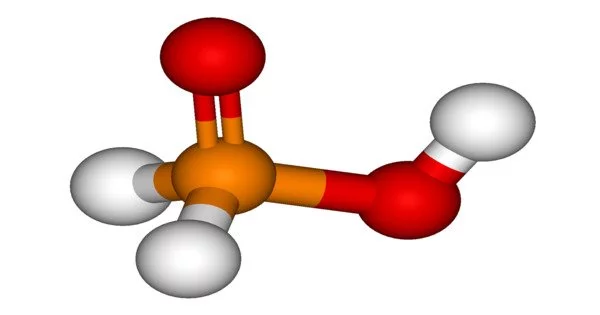
Hypophosphorous acid (HPA), also known as phosphinic acid, is a phosphorus oxyacid with the molecular formula H3PO2. It has three hydrogen atoms, one phosphorus atom, and two oxygen atoms, according to the chemical formula. It is a colorless compound with a low melting point that is soluble in water, dioxane, and alcohols. The general formula for this acid is H3PO2, but a more descriptive presentation is HOP(O)H2, which emphasizes its monoprotic nature. Hypophosphites are salts formed from this acid.
HOP(O)H2 coexists with the minor tautomer HP(OH)2 in equilibrium. The minor tautomer is sometimes referred to as hypophosphorous acid, while the major tautomer is referred to as phosphinic acid.
Properties
Hypophosphorous acid is a colorless and odorless liquid that is usually stored and handled in concentrated solutions due to its strong reducing properties. However, it can decompose over time, forming phosphine gas (PH3), which is toxic and flammable. Therefore, proper storage and handling precautions are essential when working with hypophosphorous acid.
- Chemical formula: H3PO2
- Molar mass: 66.00 g/mol
- Appearance: colorless, deliquescent crystals or oily liquid
- Density: 1.493 g/cm3; 1.22 g/cm3 (50 wt% aq. solution)
- Melting point: 26.5 °C (79.7 °F; 299.6 K)
- Boiling point: 130 °C (266 °F; 403 K) decomposes
- Solubility in water: miscible
- Solubility: very soluble in alcohol, ether
- Acidity (pKa): 1.2
- Conjugate base: Phosphinate
- Molecular shape: pseudo-tetrahedral
Preparation and availability
Hypophosphorous acid was first prepared in 1816 by the French chemist Pierre Louis Dulong (1785–1838).
The acid is prepared industrially via a two-step process: Firstly, elemental white phosphorus reacts with alkali and alkaline earth hydroxides to give an aqueous solution of hypophosphites:
P4 + 4 OH− + 4 H2O → 4 H2PO−2 + 2 H2
Any phosphites produced in this step can be selectively precipitated out by treatment with calcium salts. The purified material is then treated with a strong, non-oxidizing acid (often sulfuric acid) to give the free hypophosphorous acid:
H2PO−2 + H+ → H3PO2
HPA is typically delivered as a 50% aqueous solution. Anhydrous acid cannot be obtained simply by evaporating water because the acid readily oxidizes to phosphoric acid and phosphorous acid and also disproportionates to phosphoric acid and phosphine. Continuous extraction of aqueous solutions with diethyl ether can yield pure anhydrous hypophosphorous acid.
Applications
Metal salts are reduced back into bulk metals using hypophosphorous acid (and its salts). It is effective for a variety of transition metal ions (including Co, Cu, Ag, Mn, and Pt), but it is most commonly used to reduce nickel. This is the foundation of electroless nickel plating (Ni-P), the single most important industrial application of hypophosphites. It is primarily used as a salt (sodium hypophosphite) in this application.