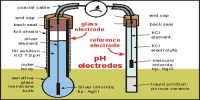
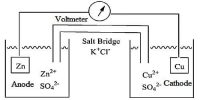
Adjusting the temperature, pressure and concentration of reactants can therefore have a large effect on the position of equilibrium. Le Chatelier’s principle is applied to industrial processes to maximise the yield of the desired product.
Haber’s Process
This reaction is very important in industry and produces ammonia as follows:
N2 (g) + 3H2 (g) = 2NH3 (g), ΔH = -92 kJ.mol-1
Increasing the pressure to 250 atm forces the equilibrium to shift to the right (the side with the smaller number of moles in the reaction equation), with effect to produce more of the desired product, ammonia.
However, it is a more complex decision to select the best temperature for this reaction. Le Chatelier’s Principle predicts that more ammonia will be produced at low temperatures, as the reaction is exothermic. Although this is true, the reaction is so slow when cold, that it takes significant amount of time to reach the equilibrium.
Use of catalysts: Hence, the reaction is usually done at a medium temperature (450°C) with the additional help from an iron catalyst to help speeding up the reaction. By definition, a catalyst is a substance that increases the rate of a reaction but is not consumed by it. Moreover, a catalyst does not affect the position of equilibrium.