Hydrogen is a clean fuel that produces only water when burned in a fuel cell. Natural gas, nuclear power, biomass, and renewable energy sources such as solar and wind can all be used to produce hydrogen. These characteristics make it an appealing fuel option for transportation and power generation applications. It can be used in cars, in houses, for portable power, and in many more applications.
A new, more energy-efficient method of producing hydrogen gas from ethanol and water has the potential to make clean hydrogen fuel a more viable alternative to gasoline for powering automobiles.
Researchers at Washington State University created pure compressed hydrogen using an ethanol-water mixture and a small amount of electricity in a novel conversion system. Because of the breakthrough, hydrogen could be produced on-site at fueling stations, requiring only the ethanol solution to be transported. It is a significant step toward eliminating the need to transport high-pressure hydrogen gas, which has been a major impediment to its use as a clean energy fuel.
“This is a new way of thinking about how to produce hydrogen gas,” said Su Ha, professor in the Gene and Linda Voiland School of Chemical Engineering and Bioengineering and corresponding author on the paper published in the journal, Applied Catalysis A. “If there are enough resources, I think it has a really good chance of making a big impact on the hydrogen economy in the near future.”
Using hydrogen as a car fuel is a promising but unrealized source of clean energy. A hydrogen fuel-cell powered car, like an electric vehicle, emits no harmful carbon dioxide. Unlike an electric car, it can be filled with hydrogen gas at hydrogen fueling stations in minutes.
This is a new way of thinking about how to produce hydrogen gas. If there are enough resources, I think it has a really good chance of making a big impact on the hydrogen economy in the near future.
Professor Su Ha
Despite hydrogen technology’s promise, storing and transporting high-pressure hydrogen gas in fuel tanks poses significant economic and safety challenges. Because of the difficulties, there is little hydrogen gas infrastructure in the United States, and market penetration of the technology is very low.
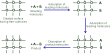
The WSU researchers developed a conversion system that included an anode and a cathode. They were able to electrochemically produce pure compressed hydrogen by injecting a small amount of electricity into an ethanol and water mixture with a catalyst. The reaction’s carbon dioxide is captured in liquid form.
Instead of transporting hazardous hydrogen gas, the conversion method would allow the existing ethanol infrastructure to be used, and compressed hydrogen gas could be easily and safely created on-demand at gas stations.

“We’re already using ethanol-containing gasoline at every gas station,” said Ha. “You can imagine that an ethanol water mixture can be easily delivered to a local gas station using our existing infrastructure, and then using our technology, you can produce hydrogen that is ready to pump into a hydrogen fuel cell car. We don’t need to worry about hydrogen storage or transportation at all.”
The electrochemical system developed by the team uses less than half the electricity of pure water splitting, another method studied by researchers for de-carbonized hydrogen production. Instead of working hard later in the process to compress the hydrogen gas, the researchers used less energy by compressing the liquid ethanol mixture, producing an already compressed hydrogen gas.
“The presence of ethanol in water changes the chemistry,” said Wei-Jyun Wang, a graduate student and co-lead author on the paper. “Our reaction can actually be carried out at a much lower electrical voltage than is typically required for pure water electrolysis.”
In addition, unlike other water splitting methods, their system does not necessitate the use of an expensive membrane. The hydrogen produced by the electrochemical reaction is then ready for use.
“A process that offers a low-electrical energy cost alternative to water electrolysis and can effectively capture carbon dioxide while producing compressed hydrogen could have a significant impact on the hydrogen economy,” said Jamie Kee, a Voiland School postdoctoral researcher and one of lead authors on the paper. “It’s really exciting because there are so many factors that go into improving hydrogen production methods.”
The researchers are working to scale up the technology and make it available on a continuous basis. They are also working on utilizing the carbon dioxide captured in the liquid. The Gas Technology Institute and the US Department of Energy’s RAPID Manufacturing Institute funded the research.