Fusion
To transform a substance from solid to liquid by applying heat is called fusion. The definite temperature at which a solid starts to melt is called its melting point. This temperature remains unchanged until all of the substance melts.
Vaporization
The phenomenon of transformation of a liquid from its liquid state to gaseous state is called vaporization. This vaporization may occur in two processes-
- Evaporation
- Boiling
Evaporation: The process in which a liquid at any temperature slowly changes from its free surface into vapor state is called evaporation.
The water has been converted into vapor even at the room temperature and for this the amount of water has been lessened. This is called evaporation.
Boiling: The process in which a liquid is rapidly converted into vapor by increasing its temperature through application of heat is called boiling. The temperature at which boiling of liquid begins is called the boiling point of that liquid. The value of boiling point depends on pressure.
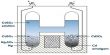
Condensation:
The process of converting a gaseous substance from its gaseous state to liquid state by lowering the temperature is called condensation.