Pi (π) bond: When two orbitals of two atoms overlap sidewise to form a covalent bond, the resulting bond is known as π-bond. It is formed only after a sigma bond is formed. Pi (π) bond is weaker than sigma (σ) bond.
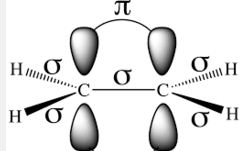
Pi (π) bond: When two orbitals of two atoms overlap sidewise to form a covalent bond, the resulting bond is known as π-bond. It is formed only after a sigma bond is formed. Pi (π) bond is weaker than sigma (σ) bond.