Scientists from the Harvard Stem Cell Institute at Boston Children’s Hospital have discovered in mice that it may be possible to repair a chronically diseased liver by forcing mature liver cells to revert to a stem cell-like state.
The researchers, led by Fernando Camargo, Ph.D., made this discovery while investigating whether a biochemical cascade known as Hippo, which regulates the size of the liver, also influences cell fate. Switching off the Hippo-signaling pathway in mature liver cells results in extremely high rates of dedifferentiation, according to an unexpected answer published in the journal Cell. This means that the cells reverse time and become stem cells again, allowing them to give rise to functional progenitor cells capable of regenerating a diseased liver.
Leprosy is one of the world’s oldest and most persistent diseases, but the bacteria that cause it may have an unexpected ability to grow and regenerate a vital organ. Scientists have discovered that parasites associated with leprosy can reprogrammed cells in adult animals to increase the size of the liver without causing damage, scarring, or tumors.
The findings suggest that humans could adapt this natural process to renew aging livers and increase healthspan (the amount of time they live disease-free). According to experts, it may also aid in the regeneration of damaged livers, reducing the need for transplantation, which is currently the only curative option for people with end-stage scarred livers.
If we can identify how bacteria grow the liver as a functional organ without causing adverse effects in living animals, we may be able to translate that knowledge to develop safer therapeutic interventions to rejuvenate aging livers and regenerate damaged tissues.
Professor Anura Rambukkana
Previous research encouraged the regrowth of mouse livers by generating stem cells and progenitor cells – the step after a stem cell that can become any type of cell for a specific organ – using an invasive technique that frequently resulted in scarring and tumor growth.
To overcome these negative side effects, Edinburgh researchers built on their previous discovery of the partial cellular reprogramming ability of the leprosy-causing bacteria, Mycobacterium leprae.
Working with the US Department of Health and Human Services in Baton Rouge, Louisiana, the team infected 57 armadillos with the parasite and compared their livers to those of uninfected armadillos and those found to be resistant to infection.
They found that the infected animals developed enlarged – yet healthy and unharmed – livers with the same vital components, such as blood vessels, bile ducts, and functional units known as lobules, as the uninfected and resistant armadillos.
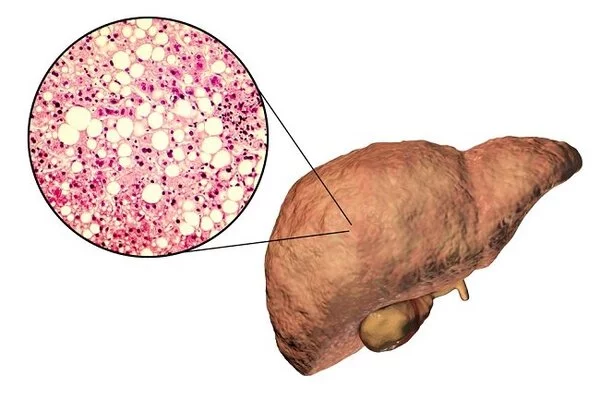
The team believe the bacteria ‘hijacked’ the inherent regenerative ability of the liver to increase the organ’s size and, therefore, to provide it with more cells within which to increase. They also discovered several indicators that the main kinds of liver cells – known as hepatocytes – had reached a “rejuvenated” state in the infected armadilllos. Livers of the infected armadillos also contained gene expression patterns — the blueprint for building a cell – similar to those in younger animals and human fetal livers.
Genes associated with metabolism, growth, and cell proliferation were activated, while those associated with aging were downregulated, or suppressed. Scientists believe this is because the bacteria reprogrammed the liver cells, returning them to an earlier stage of progenitor cells, which then became new hepatocytes and grew new liver tissues.
The researchers hope that the discovery will aid in the development of human interventions for aging and damaged livers. Liver diseases currently kill two million people worldwide each year.
The research was published in the journal Cell Reports Medicine. The Medical Research Council in the United Kingdom, as well as the National Institutes of Health and the National Institute of Allergy and Infectious Diseases in the United States, funded this research.
“If we can identify how bacteria grow the liver as a functional organ without causing adverse effects in living animals,” said lead author Professor Anura Rambukkana of the University of Edinburgh’s Centre for Regenerative Medicine, “we may be able to translate that knowledge to develop safer therapeutic interventions to rejuvenate aging livers and regenerate damaged tissues.”