A little leeway may be just what the doctor ordered. According to new research, peptides, which are short chunks of protein used to treat Type 2 diabetes, may be more effective if they can move back and forth between different shapes. The findings could aid in the development of new diabetes drugs as well as other therapeutic peptides.
More broadly, the discovery contradicts popular belief that the body’s molecular signaling machinery is based on having a single ideal and static partner to activate cellular receptors. The machinery of life may be more dynamic than previously thought.
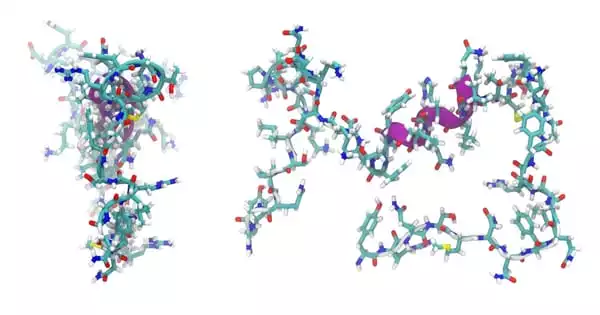
GLP-1, the peptide, was previously known to have a rigidly helical, corkscrew shape. A peptide engineered to form a sudden kink near its end better activated its cellular target, which promotes insulin release from the pancreas, when compared to a peptide locked into this helical shape. GLP-1 is likely to be able to switch back and forth between these two forms in the body, maximizing its potency.
“I believe most molecular scientists imagine this peptide bound to the receptor as having a single ideal shape,” says Sam Gellman, a chemistry professor at the University of Wisconsin-Madison who oversaw the new study. “And what we mean is that this ideal interaction between these two units is probably overly simplistic. That peptide must remain mobile in specific ways in order to be effective.”
We usually envision a single idealized structure that we are attempting to achieve. However, based on these findings, I believe that the best way to be most effective is to maintain specific modes of flexibility. With that in mind, you’re looking at the molecule in different ways.
Professor Sam Gellman
Gellman and colleagues published their findings in the journal Nature Chemical Biology. Brian Cary was a doctoral student in Gellman’s lab at the time and led the research.
Many hormones, including insulin and GLP-1, are peptides. These peptides provide critical information to cells that affect metabolism, such as blood sugar control. Peptides communicate this information by binding to and activating specialized receptor proteins on the cell’s surface.
Biologists frequently envision the peptide as a key that fits into and unlocks the receptor’s lock. A peptide, like a key, requires the proper shape to function properly. Drug developers frequently try to change the shape of a peptide in order to make it a better drug. Because GLP-1 has a corkscrew shape, it was assumed that making the peptide more helical would improve GLP-1’s ability to activate its target in the body.
Cary discovered that when he engineered GLP-1-like peptides to better form this corkscrew shape, they were less potent. Cary designed and created a series of different shaped GLP-1 varieties to test in order to investigate this unexpected finding. Cary was able to create two types of shapes by using amino acids that are not normally found in natural peptides. One category was helical all the way through, while the other bent at a sharp angle near one end.
When the researchers tested the various shapes, they discovered a puzzle: helical peptides bound strongly to the receptor but were terrible at activating it; kinked proteins bound weakly but effectively activated the receptor when they finally docked.
To solve this conundrum, the researchers devised a new model of how GLP-1 might function. GLP-1 binds to and activates its target as a helix — the correct shape key to fit in the lock, according to this model. GLP-1 can then switch to a new shape with a kink near the end. The kink aids in the reset of GLP-1’s cellular target, preparing it to send a new signal. The peptide can then switch back to a helix to fully dock and activate the target again.
“By going back and forth but never completely disconnecting from the receptor, you get to keep signaling and be more effective as a signal-inducing peptide,” Gellman explains. Only a peptide able to switch back and forth can accomplish this feat.
Data showing a GLP-1-like peptide bound to its receptor in two different shapes supported this model. Cryo-EM, or molecular-level imaging of protein shapes, enables scientists to see how biological machinery fits together to function.
“Seeing that Cryo-EM structure and recognizing that there are two states was seeing strong evidence that there is a second state that plays a functional role here,” Gellman says. In the future, Gellman advises drug developers to consider whether their preferred peptides would benefit from the ability to take on multiple shapes.
“We usually envision a single idealized structure that we are attempting to achieve. However, based on these findings, I believe that the best way to be most effective is to maintain specific modes of flexibility “he claims. “With that in mind, you’re looking at the molecule in different ways.”