Chromic oxide is mixed with powdered Aluminium in the ratio 3:1 and is placed in a large fire clay crucible. A mixture of barium peroxide and Mg powder is placed over this. The crucible is surrounded by sand which prevents loss of heat by radiation. The mixture is ignited by a piece of Mg ribbon. During this process a large amount of heat is liberated, in which Cr2O3 is reduced to chromium.
The molten chromium is collected in the crucible and aluminium oxide is removed as slag.
Cr2O3 + 2Al →2Cr + Al2O3 + 468.6 kJ
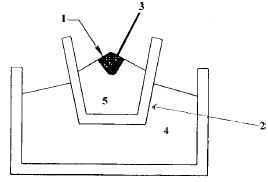
Here,
- BaO2 + Mg Powder
- Fireclay crucible
- Magnesium ribbon,
- Sand
- Cr2O3 + Al










