Second order reaction: Formation of Cobalt (II) chloride from Carbon Monoxide
A reaction is assumed to be the second order if its rate is determined by the deviation of two concentration terms or rate of the reaction is proportional to the second power of the concentration of a solitary essence. A second order reaction is a type of chemical reaction that depends on the concentrations of one second order reactant or on two first order reactants. As an example of the first type, the reaction NO2 + CO → NO + CO2 is second-order in the reactant NO2 and zero order in the reactant CO.
Explanation
Formation of Cobalt (II) chloride (COCl2) from CO and Cl2 according to the equation-
CO (1 vol) + Cl2 (1 vol) → COCl2 (1 vol)
is a second order gaseous reaction. Since the reaction proceeds with a change in volume the extent of the reaction can he followed by measuring the change in pressure at constant volume and constant temperature. Some kinetic data are shown in Table.
Table: Formation of Cobalt (II) chloride COCl2
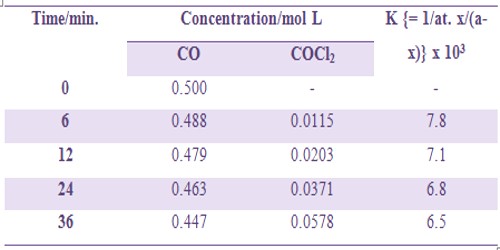
The slight deviation of the values of k indicates complex mechanism of the reaction.