Semi-permeable Membrane: Vapour pressure theory
A membrane that allows only the solvent molecules to pass through it, but not the solute molecules is known as a semi-permeable- membrane. Natural membranes are mostly semi-permeable. For examples, skin inside the egg shell, membranes around the red blood corpuscle, animal bladder, and vegetable tissue are all semi-permeable.
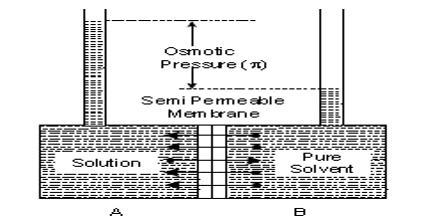
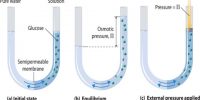
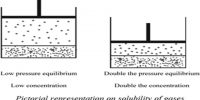
Vapour pressure theory: The vapour pressure theory assumes that neither the solute nor the solvent molecules can pass through the tiny holes or capillaries present in the membrane. Thus the capillaries have pure solvent on one side and solution on the other side with a ‘finite gap’ separating them. Since the vapour pressure of the solution is lower than that of the pure solvent, the diffusion of vapour from the solvent side to the solution side will occur across the finite gap. It is thus assumed that only the molecules in the vapour phase can pass through the membrane. The vapour pressure theory can explain the mechanism of osmosis in most cases.