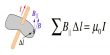State of a system of Thermodynamics
Values of those quantities which determine the condition of the system are called thermodynamic coordinates of the system or variables of the system. As an example, the state of an electric battery requires the specification of the amount of electric charge it contains. The thermodynamic state of a system is defined by specifying values of a set of measurable properties sufficient to determine all other properties. For fluid systems, typical properties are pressure, volume, and temperature. More complex systems may require the specification of more unusual properties. For example, gas confined in a cylinder is a system and the characteristics of the state of the gas are denoted by its pressure, volume, and absolute temperature. So, pressure, volume, and absolute temperature are called thermodynamic coordinates.
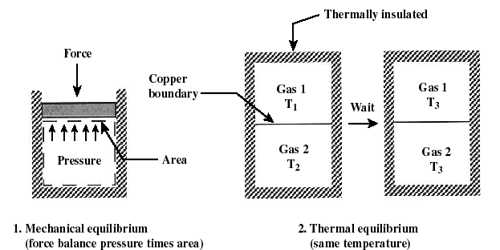
A thermodynamic system is a quantity of matter of fixed identity, around which we can draw a boundary. A thermodynamic state is a set of values of properties of a thermodynamic system that must be specified to reproduce the system. The boundaries may be fixed or moveable. Work or heat can be transferred across the system boundary. Everything outside the boundary is the surroundings.