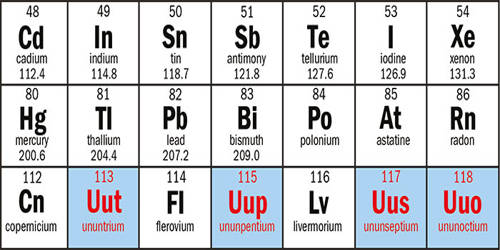
The first superheavy element is Element 113, which was recently discovered by the consolidation of scientists from the Lawrence Livermore National Laboratory and the Russian Institute for Nuclear Research. Like some heavy elements, aerospace components are artificially produced in cyclotron experiments. In chemistry, super heavy ingredients, also known as transaction elements, transactinide or ultra-heavy elements, are chemical elements with more than 103 atomic numbers. Further steps in this decay chain, one of the nuclei produced, isotope laranium 266 – a nucleus in which 103 protons and 163 neutrons have never been seen before. Isotopes previously known as laurencium have fewer neutrons and are less stable. The extracellular components are located immediately outside the actinides in the phase table; the heaviest actinide is Laurensium (atomic number 103).
But during this talk held in January at the commencement of the International Year of the Periodic Table, Agnesian said that the discovery of ultraviolet elements (SHEs) with 104 and above proton numbers was like opening a Pandora’s box. He says many unexpected problems are more difficult to solve than creating SHE in the first place. Glenn T. Sieberg first proposed the actinide concept, which led to the adoption of the actinide series. He also proposed a transactinide series spanning the 104 to 121 element and the super actionized series from about 122 to 153 (although more recent work instead suggested the completion of the supractipside series in the 157 elements). At one time it had only 83 naturally present ingredients, beginning with hydrogen and ending with uranium. These elements have a half-life that is comparable to the age of the Earth, which is about 4.5 billion years old. Since the 1940s, physicists have been able to create unstable materials that can cause the loss of light elements in a timescale that can range from thousands of years to tiny fractions of a second. Except for Rutherfordium and Dubonium, even long-lasting isotopes of extracellular material have a half-life of minutes or less. 102-109 of the elements involved in the controversy over the naming of the material. Some of these ingredients use regular names for several years after their discovery is confirmed.
Super heavy magic nuclei can have interesting shapes that give stability, such as a so-called bubble configuration with a hole in the middle. “These have never been discovered yet, but the area they are now looking for is really on the edge of the bubble region,” Nazarivicz said. None of these components have ever been collected in macroscopic specimens. Superheavy elements are named after physicists and chemists or important locations involved in the synthesis of components.
If an island of stability does exist, there is no limit to how long its nuclei may last. They could turn out to be stable enough to be found in nature, albeit in such small quantities that we have not seen them yet. IUPAC determines the existence of a component if its lifespan is longer than 10-15 seconds, which takes time for the nucleus to form an electronic cloud. Although nothing has been discovered so far, scientists are hoping the evidence for the island of sustainability is at one corner or the other.