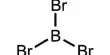
Scientists have succeeded in creating what is expected to be an allotrop form of “black nitrogen” based on observations of comparative elements which made them stubborn for decades.
As the technology improved, chemists observed that the mills were more visible than they first appeared. For example, when placed under great pressure elements near the top of the table they will adopt similar structures by placing their column down under more general conditions. The only exception is nitrogen, which strongly refuses to mimic phosphorus no matter how much pressure is applied. A paper in Physical Review Letters has changed the description of the crystal state of nitrogen which is similar to that of black phosphorus.
This was long before we discovered the cause of these similarities – the electrons in the “shells” orbit and each element in a column has an outer shell each row with the same number of electrons representing the extra shell. Periodic tables were necessary for chemistry to become a true science, based on grouped elements that behave similarly in columns in order to predict their behavior.
Phosphorus is a versatile element commonly found in two main forms, in red and highly volatile white allotropes. When heated under pressure, however, white phosphorus converts to the most stable structure, known as black phosphorus, which has some resemblance to the sheet graphite of zigzagging atoms. Arsenic and antimony under the table can take similar forms.
Dominic Laniel, a doctor at Bayerut University, applied nitrogen at 4,000 degrees Celsius (2008F) at atmospheric pressure of 1.4 million atmospheres. No, but Laniel and co-authors bombarded the product with X-rays to learn its structure.
Laniel said in a statement, “We were surprised and excited to see the measurement data and suddenly provide features of our black phosphorus structure.” Subsequent experiments and calculations have confirmed this discovery. This means there is no doubt about it: nitrogen is not actually an exceptional element, but follows the same golden rules of the periodic table as carbon and oxygen.
Studying new primary forms can often improve our understanding of them. In this light, the surprise-component of two-dimensional layers of black nitrogen may have some value in matching graphene. Although nitrogen is in abundant supply, the conditions required for it to be black are so extreme that direct use of the new allotrope is unlikely.