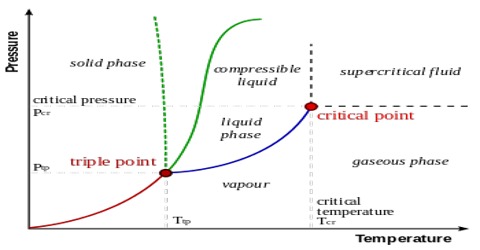
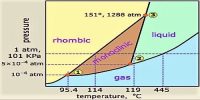
The triple points:
P is the point at which the curves AP, PQ and RP meet and the three phases rhombic, monoclinic and sulphur vapour co-exist. This is, therefore, a triple point. The temperature and pressure at this point are 95.50C and 1 x 10-5 atmosphere respectively.
Q is a triple point where monoclinic, liquid and sulphur vapour co-exist as the curves PQ, QB and RP meet. The temperature and pressure at this point are 119.3°C and 6 x 10-3 atmosphere respectively.
R is the point at which the curses PR, QR and RT meet. So this is the point in the diagram where rhombic, monoclinic and liquid sulphur co-exist. The temperature and pressure which define this point are 151°C and 1290 atmosphere respectively.
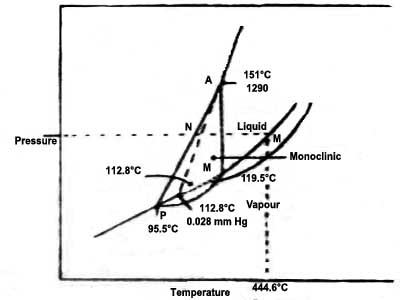
S is the point at which the curves PS (extension of AP), QS (extension of BQ) and RS (extension of TR) meet. All the three curves show the metastable equilibria between phases as indicated. This also is a triple point at which rhombic, liquid and sulphur vapour can co-exists in a metastable equilibrium. The temperature and pressure of this metastable triple point are 112.80C and 0.028 mm at Hg.
It may be noticed that P and Q lie at pressures below the atmospheric pressure. When rhombic sulphur is gradually heated at one atmosphere pressure it will turn into the monoclinic form at around 1150C, and if the heating is continued the monoclinic form starts liquefying once the temperature tracks the value indicated by M. When it is completely in the liquid form temperature may be increased until the point N is reached. The liquid and vapour are in equilibrium at this temperature and is the boiling point (444.60C) of sulphur at one atmosphere pressure.