The postulates of valence bond theory (VB Theory)
1) The central metal atom/ion makes available a number of vacant orbitals equal to its coordination number.
2) These vacant orbitals form covalent bonds with the ligand orbitals.
3) A covalent bond is formed by the overlap of a vacant metal orbital and filled ligand orbitals. This complete overlap leads to the formation of a metal ligand, σ (sigma) bond.
4) A strong covalent bond is formed only when the orbitals overlap to the maximum extent. This maximum overlapping is possible only when the metal vacant orbitals undergo a process called ‘hybridisation’. A hybridised orbital has a better directional characteristics than an unhybridised one.
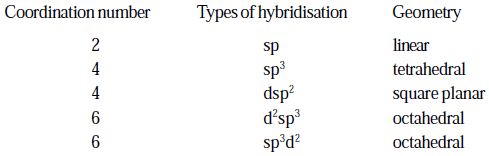
The following table gives the coordination number, orbital hybridisation and spatial geometry of the more important geometrics.