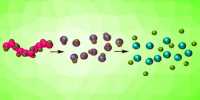
Lithium and oxygen are the ingredients for anything close to a perfect battery, in theory. Unfortunately, the effort to make these a reality has so far resulted in something that could lose performance very quickly. New research suggests an answer to this, but with a twist. Whether your phone is recharged or power is supplied to a country at night, the need for a better method of conserving electricity is so great that dozens of potential technologies are being explored.
Lithium-oxygen combinations seem invincible in many respects: they are now theoretically capable of storing at least five times the amount of charge per lithium-ion battery, which now dominates the small to medium scale market. Lithium and oxygen are also so light that their combination will beat any weight alternative, which is important for portable use. It is not like a short supply of oxygen.
The problem is that batteries require electrodes, and lithium-oxide anodes have quickly eroded the cathodes of marketing systems to consider mass-market applications. Linda Nazar, a professor at the University of Waterloo in Canada, believes the answer is in favor of lithium peroxide (Li2O2) and lithium superoxide (LiO2) in favor of lithium oxide (Li2O).
Nazar writes in Science, “Lithium oxide (Li2O) is chemically less reactive with organic solvents than peroxides and superoxide.” This idea has been seen before by people, but batteries refuse to play ball, producing lithium peroxide when discharged without doing anything more harmful. Nazar calculates that the room temperature is in favor of lithium-peroxide production, so he runs the battery at 150ºC (302ºF). Not only did it work but it was about 100 percent efficient in terms of discharge ratio in charge.
Maintaining such high temperatures imposes an energy expenditure on the system. Surely no one wants something hot in their pocket; they even have a laptop, so there are a lot of apps out there. In addition to these obvious problems, Nazar faces all the usual hurdles in reaching the prototype stage from the battery lab bench.
However, the potential for Nazar’s products in electric vehicles with a density of four times higher than that of lithium-ion batteries is already huge. In addition, the cathode of the tested model was made from nickel – a wide lot of metal. The high cobalt content in most existing battery cathodes is becoming a problem as price sparks and metals fuel war and demand for environmental destruction.