Weston Standard Cell
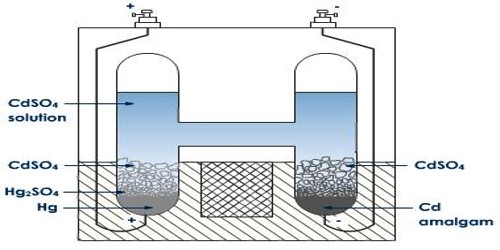
The accuracy of measurement of e.m.f of a cell by the Poggendorf method depends on the constancy of the potential of the standard cell, provided the galvanometer used is a sensitive one. A standard cell will maintain a constant and reproducible e.m.f. provided that very little current is drawn from it. Another condition is that the e.m.f should be affected by temperature only slightly. The standard cell usually employed in potentiometric measurements is the Weston cadmium cell, shown in Figure.
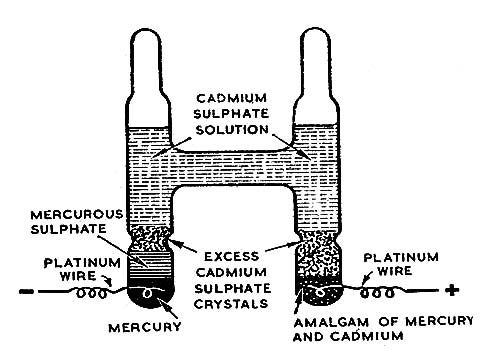
Fig: Standard Weston Cell
One limb of the H-shaped vessel contains mercury over which is placed a paste of mercurous sulphate and mercury. In the other limb is placed a Cadmium amalgam. The rest of the vessel is filled with a Saturated solution of sulphate containing an excess of solid CdSO4, 8/3 H2O crystals. The reaction taking place in the cell when it is producing current i-
Cd(s) + Hg2SO4 (s) + aq → CdSO4.8/3 H2O (s) + 2Hg (I)
The potential of the cell is 1.0183 V at 250 C and it has a very small temperature coefficient.