STP :
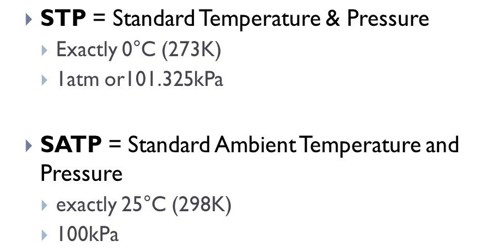
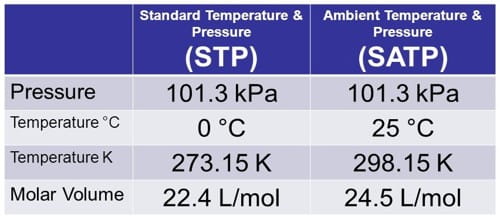
The full name of STP is Standard Temperature and Pressure. In this method temperatures and pressures are considered as 0°C or 273 K and 1 atm respectively and molar volume is 22.4 liters/mole.
Standard Temperature and Pressure (STP) is defined by IUPAC (International Union of Pure and Applied Chemistry) as air at 00 C (273.15 K, 320 F) and 105 pascals (1 bar). STP is usually used to define standard conditions for temperature and pressure which is vital for the measurements and certification of chemical and physical procedures. It is regularly used in the Imperial and USA system of units – as air at 600F (5200 R, 15.60 C) and 14.696 psia (1 atm, 1.01325 bara)
- also named “1 Standard Atmosphere”
- At these conditions, the volume of 1 mol of gas is 23.6442 liters.
- These conditions are the most normally used to define the volume term Sm3 (Standard cubic meter)
- Standard Temperature, Pressure: 273.15K (0°C), 101.3kPA.
SATP :
The full name of SATP is Standard Ambient Temperature and Pressure. In this method temperature and pressure are considered as 25°C or 298 k and 102 K Pa respectively and molar volume is 24.789 liters.
Standard Ambient Temperature and Pressure (SATP) is a reference with the temperature of 250 C (298.15 K) and pressure of 101.325 kPa. Anyway, STP is standard temperature and pressure, which is 273.15 kelvin (or 0 degrees Celsius) and a little less than 1 atm of pressure.
At these conditions, the volume of 1 mol of gas is 24.4651 liters.
Standard Ambient Temperature, Pressure: 298.15K (25°C), 101.3kPA.
STAP is a standard ambient temperature and pressure. The dissimilarity is the temperature. Most of the time it is easier for experimenters to have their circumstances at room temperature. So STAP is 298.15 kelvin (or 25 degrees Celsius) and again a pressure of 1 atm.